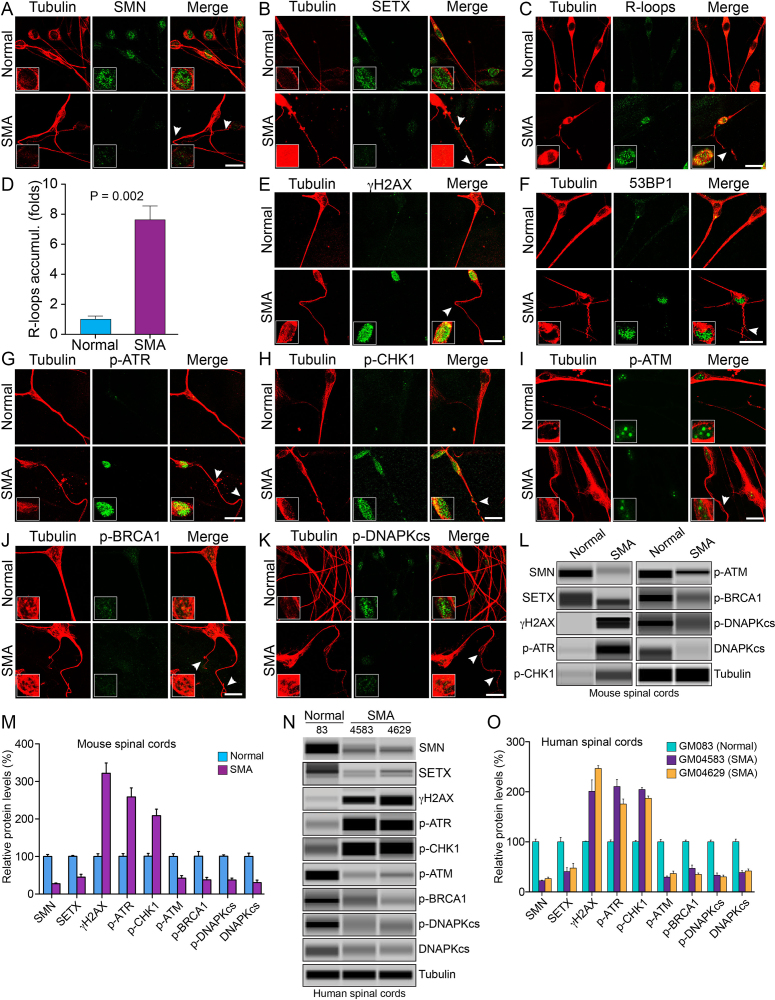
Figure 6.
Chronic low levels of SMN cause SETX-deficiency, accumulation of R-loops and activation of DDR pathways in spinal cord neurons derived from SMA mice, and spinal cord tissues from SMA mice and SMA type I patients. Primary motor neurons were cultured using spinal cords isolated from 7-day-old wild-type (Normal) and SMAΔ7 mice (SMA). The spinal cord ex-plants were cultured in vitro for 12–14 days to allow differentiation and growth of motor neurons in laminin-coated 8-well chambers, fixed with 4% PFA and stained with antibodies and IF examined by confocal microscopy. (A) Low levels of SMN (green) in SMA neurons causes axonal defects as shown by staining with neuron-specific β-tubulin-III (clone TUJ1) (red). Axonal defects include bending, folding, retraction and ballooning of axons (arrowheads) that indicate degeneration of SMN-deficient neurons. Insets show a magnified view of a neuron cell body with the nucleus. (B) Staining for SETX (green) and β-tubulin-III (red) show reduced staining of SETX. (C) Staining of neurons with antibody (S9.6) against RNA–DNA hybrids (R-loops) (green) and β-tubulin-III (red) show increased accumulation of R-loops in SMA neurons compared to normal neurons. (D) Quantitative (mean ± s.e.m., n = 3) and statistical analysis (unpaired t-test) shows marked increase (7.62 ± 0.97, P = 0.002)-fold in accumulation of R-loops in SMA neurons compared to non-SMA (Normal) neurons. Staining for markers of DDR and DNA repair pathways: (E) β-tubulin-III (red) and phospho-H2AX (γH2AX) (green) and (F) β-tubulin-III (red) and 53BP1 (green) show increase in phosphorylation of H2AX and accumulation of 53BP1 foci in SMA neurons compared to normal neurons that suggest accumulation of DSB-related DNA damage in SMA motor neurons. (G) Staining of neurons with β-tubulin-III (red) and phospho-ATR (p-ATR) (green); (H) β-tubulin-III (red) and phospho-CHK1 (p-CHK1) (green), downstream target of ATR. (I) Neurons stained with β-tubulin-III (red) and phospho-ATM (p-ATM) (green); (J) β-tubulin-III (red) and phospho-BRCA1 (p-BRCA1) (green); (K) phospho-DNA-PKcs (p-DNAPKcs) (green) and β-tubulin-III (red). Scale bar is 20 μm. (L) Protein extracts were prepared from the spinal cords isolated from 7-day-old non-SMA (Normal) and SMA mice, and examined by IB analysis using automated capillary-based western blot system (ProteinSimple). Representative capillary-blot images of proteins and phospho-proteins are shown (full-length blots are included inSupplementary Figures S12 and 13). IB analysis of proteins in tissue lysates of the spinal cords isolated from 7-day-old non-SMA (Normal) and SMA pups shows increase in levels of phospho-H2AX (γH2AX) and phospho-ATR (p-ATR) in SMA mice compared to normal mice. (M) Quantitative (mean ± s.e.m., n = 3 mice/group) and statistical analysis (unpaired t-test) of IB data from mouse spinal cords show that SMN and SETX levels reduced to (27.67 ± 1.870%, P = 0.000) and (45.30 ± 7.66%, P = 0.002), respectively, in SMA mice (SMA) compared to non-SMA (Normal) mice. Analysis of DDR markers shows increase in levels of γH2AX (3.21 ± 0.27-fold, P = 0.001), p-ATR (2.58 ± 0.24-fold, P = 0.003) and p-CHK1 (2.08 ± 0.17-fold, P = 0.004). DSB repair markers of HR, p-ATM is reduced to (42.35 ± 6.94%, P = 0.004) and p-BRCA1 reduced to (37.85 ± 6.50%, P = 0.011) in SMA mice (SMA). DSB repair marker of NHEJ, phospho-DNA-PKcs (p-DNAPKcs) reduced to (37.47 ± 4.86%, P = 0.000) and total DNA-PKcs reduced to (30.54 ± 6.74%, P = 0.004) in SMA mice. (N) IB analysis of proteins in tissue lysates of human spinal cords (lumbar region) isolated from age matched non-SMA, GM083 (Normal) and severe SMA type I patients, GM04583 and GM04629 (SMA). (O) Quantitative and statistical analysis of IB data from human spinal cords show low levels of SMN in SMA patients, GM04583 (22.43 ± 1.07%, P = 0.000) and GM04629 (26.63 ± 2.91%, P = 0.000) compared to normal (non-SMA) human (100.4 ± 5.221%, P = 0.000) (GM083). SMA patient spinal cords show decrease in SETX levels GM04583 (59.53 ± 11.67%, P = 0.007) and GM04629 (52.52 ± 12.90%, P = 0.015) compared to normal (non-SMA) human (GM083). Analysis of DDR markers shows increase in levels of γH2AX in SMA patients (2.01 ± 0.22-fold, P = 0.011) in GM04583 and (2.46 ± 0.05-fold, P = 0.000) in GM04629, p-ATR (2.10 ± 0.14-fold, P = 0.001) in GM04583 and (1.75 ± 0.10-fold, P = 0.002) in GM04629, and p-CHK1 (2.04 ± 0.04-fold, P = 0.000) in GM04583 and (1.87 ± 0.04-fold, P = 0.000) in GM04629. Analysis of DDR and HR markers shows p-ATM reduced to (29.65 ± 2.18%, P = 0.000) (GM04583) and (36.63 ± 4.95, P = 0.000) (GM04629), and p-BRCA1 (47.09 ± 6.63, P = 0.001) (GM04583) and (35.17 ± 2.717, P = 0.000) (GM04629). Analysis of NHEJ pathway shows phospho-DNA-PKcs (p-DNAPKcs) reduced to (33.33 ± 4.512%, P = 0.000) (GM04583) and (30.06 ± 3.590, P = 0.000) (GM04629), and total DNA-PKcs (38.52 ± 3.910, P = 0.000) (GM04583) and (41.76 ±4.193, P = 0.001) (GM04629) suggesting that low levels of SMN cause deficiency and the loss of DNA-PKcs activity.