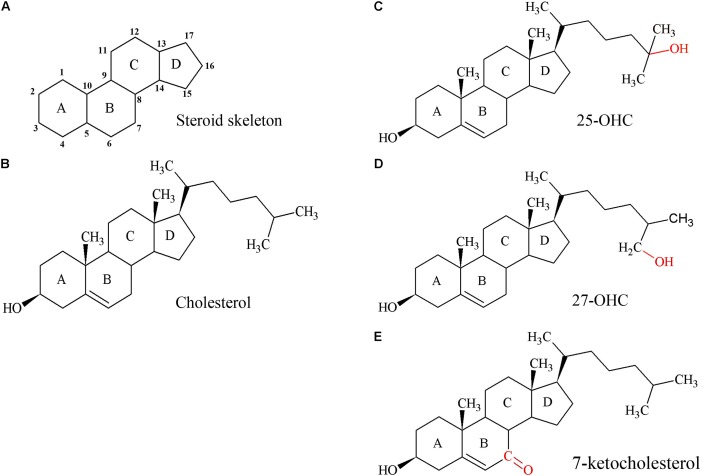
FIGURE 2.
Structures of the steroid skeleton, cholesterol, and common oxysterols. (A) All steroids have the same basic perhydro-1,2-cyclopentenophenanthrene skeleton. Letters designate each ring, the carbon atoms are numbered. A slight variation in this skeleton or the introduction of functional groups result in various classes of steroids. (B) Unesterified cholesterol contains this skeleton with a hydroxyl group, two methyl groups, and a hydrogen tail. In the esterified form, a fatty acid would be bound to the hydroxyl group by an ester bond. (C) 25-hydroxycholesterol (25-OHC), the most extensively studied oxysterol. (D) Oxysterol 27-hydroxycholesterol (27-OHC). (E) Oxysterol 7-ketocholesterol. Red molecules indicate the positions of hydroxylation (C,D) or oxidation (E) of cholesterol to 25- or 27-OHC, or 7-ketocholesterol, respectively. Oxysterols are intermediates of cholesterol catabolism and act as signaling molecules with regulatory impact on various cellular processes including lipid metabolism.