Abstract
Background
Resurveying historical vegetation plots has become more and more popular in recent years as it provides a unique opportunity to estimate vegetation and environmental changes over the past decades. Most historical plots, however, are not permanently marked and uncertainty in plot location, in addition to observer bias and seasonal bias, may add significant error to temporal change. These errors may have major implications for the reliability of studies on long-term environmental change and deserve closer attention of vegetation ecologists.
Material & Methods
Vegetation data obtained from the resurveying of non-permanently marked plots are assessed for their potential to study environmental-change effects on plant communities and the challenges the use of such data have to meet. We describe the properties of vegetation resurveys distinguishing basic types of plots according to relocation error, and we highlight the potential of such data types for studying vegetation dynamics and their drivers. Finally, we summarise the challenges and limitations of resurveying non-permanently marked vegetation plots for different purposes in environmental change research.
Results and Conclusions
Resampling error is caused by three main independent sources of error: error caused by plot relocation, observer bias, and seasonality bias. For relocation error, vegetation plots can be divided into permanent and non-permanent plots, while the latter are further divided into quasi-permanent (with approximate relocation) and non-traceable (with random relocation within a sampled area) plots. To reduce the inherent sources of error in resurvey data, the following precautions should be followed: (i) resurvey historical vegetation plots whose approximate plot location within a study area is known; (ii) consider all information available from historical studies in order to keep plot relocation errors low; (iii) resurvey at times of the year when vegetation development is comparable to the historical survey to control for seasonal variability in vegetation; (iv) keep a high level of experience of the observers to keep observer bias low; and (v) edit and standardise datasets before analyses.
Keywords: Environmental change, long-term vegetation dynamics, non-permanent plots, non-traceable plots, observer bias, pseudo-turnover, quasi-permanent plots, relocation error, semi-permanent plots, vegetation resampling
Introduction
Ecosystems are dynamic and constantly changing in response to internal mechanisms (e.g. succession) and external drivers (e.g. environmental change). Human-induced factors, particularly land-use change, atmospheric pollution and climate change, have become important drivers of change in vegetation composition and diversity over the past decades (Walther et al. 2005; Bobbink et al. 2010). Understanding the effects of the different drivers – both anthropogenic and natural – is important for an effective management and conservation of natural resources.
Vegetation dynamics are often inherently slow, and the relevant time-scale for biodiversity management often exceeds decades. Most ecological research projects are performed over short time periods (with the important exception of palaeoecological studies, e.g. Willis & Birks 2006) and translating the findings from these studies to relevant time scales is not always obvious. Long-term vegetation dynamics can best be studied by monitoring of vegetation using permanent plots. Unfortunately, long-term monitoring of permanent plots is extremely rare and mostly restricted to the last few decades (e.g. Bakker et al. 1996; Pauli et al. 2012; but see e.g. Silvertown et al. 2006). One alternative to the use of permanent plots to study long-term community change is resurveying of vegetation plots from surveys made by independent authors a period of time ago (historical plots). There is a huge amount of such data accumulated in literature, in addition to unpublished work, during the history of vegetation research (Dengler et al. 2011). However, the exact location of the historical plots is only rarely known. These non-permanently marked plots have been increasingly resurveyed in the past few decades (e.g. Persson 1980; Chytrý & Danihelka 1993; Hédl 2004; van Calster et al. 2008; van den Berg et al. 2011; Wipf et al. 2013; Koch & Jurasinski 2015) the results of which have also been used in meta-analyses (e.g. Fischer 1999; Verheyen et al. 2012; Bernhardt-Römermann et al. 2015). But using these kinds of data to document vegetation change has some non-trivial challenges.
In this paper, we highlight the opportunities that historical, non-permanently marked plots offer for the study of vegetation dynamics and point out the most important conceptual challenges of this approach. We further describe possible solutions to account for limitations in data structure and by sources of error like observer bias, phenological differences or spatial heterogeneity.
A short history of plot-based vegetation resurveying
The history of collecting vegetation data by recording all species within a restricted area (plot) goes back to the 19th century. The historical plots were commonly established for phytosociological purposes, i.e. describing in detail the variation in vegetation and species composition of a certain vegetation type or area. During the last decades of the 20th century, vegetation ecologists realised the potential of these historical plot data and started to resurvey vegetation on formerly surveyed plots in order to study vegetation shifts over time. The approach has been used, for instance, to detect upward shifts in mountain areas and northward shifts along latitudinal gradients for plant communities and species ranges (Grabherr et al. 1994; Chapin et al. 1995; Klanderud & Birks 2003; Tape et al. 2006; Kelly et al. 2008; Wilson & Nilsson 2009; Harrison et al. 2010; Felde et al. 2012; Grytnes et al. 2014); other studies have focused on vegetation changes in grasslands (e.g. Fischer & Stöcklin 1997; Bühler & Roth 2011; Van Den Berg et al. 2011), forests (e.g. Keith et al. 2009; Hédl et al. 2010; Šebesta et al. 2011, Kopecký et al. 2013), and peatlands (e.g. Gunnarsson et al. 2000; Kapfer et al. 2011; Koch & Jurasinski 2015) documenting changes in species assemblages and composition and often declines in species richness (but see Vellend et al. 2013). To give an impression of the magnitude of the worldwide compiled vegetation-plot data (including original and resurveyed plots), there are more than 3 million vegetation-plot records in current databases that have been sampled by vegetation scientists worldwide and across vegetation types, with the oldest records dating from 1864 (Schaminée et al. 2009; Dengler et al. 2011; Waller et al. 2012). The availability of these datasets from different regions and time periods bears unparalleled potential for comprehensive research to foster our understanding of vegetation dynamics, and patterns and processes over space and time, by linking local and global scales and by bridging the gap between long-term (palaeo) and short-term ecological research.
Types of plot-based historical vegetation data
As stated above, there is a vast amount of vegetation data from plots collected over the past century. We refer to these resources as ‘historical’ vegetation data regardless of the time of record. Because these data have been sampled for many different purposes, the historical vegetation data can differ greatly with respect to sampling unit (e.g. plot-data or small-area surveys), meta-data availability and quality (e.g. method description, sampling protocol), data quality (e.g. experience of the investigator) and sampling design (e.g. number of plots and their spatial distribution). Typically, these data have a plot structure where the sampled area is geographically restricted with the area of sampling units commonly ranging from about 1–103 m2 (e.g. phytosociological plots) to about 10 000–100 000 m2 (e.g. mountain summits or management compartments). The records usually consist of a complete species list and, for smaller scales, they typically provide estimates of species coverage (e.g. Du Rietz 1921; Braun-Blanquet 1964). Relatively few historical datasets are accompanied by additional information, as for instance number of individuals, biomass or local environmental variables (e.g. soil pH, soil water content, humus content).
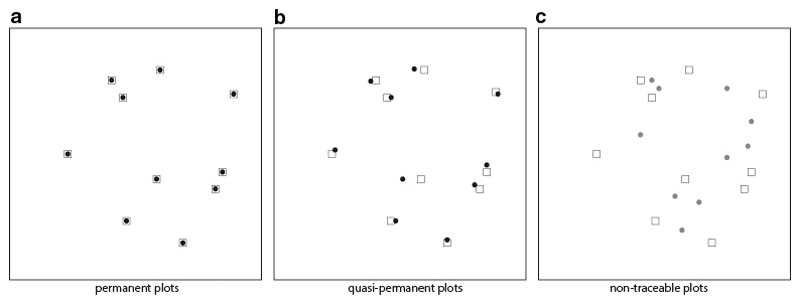
The position of the majority of historical vegetation plots has not been permanently marked in the field. However, the locations of some plots have sometimes been described and in some cases marked on detailed maps. To distinguish non-permanently marked plots from permanent ones, previous literature has referred to non-permanently marked plots as ‘semipermanent’ or ‘semi-permanent’ plots (e.g. Persson 1980; Lawesson 2000) or as ‘non-permanent’ plots (Kapfer et al. 2011; Felde et al. 2012). We suggest to distinguish between two types of non-permanently marked plots according to the detail of information on plot location available from historical studies (Fig. 1). We propose the term ‘quasi-permanent’ (Fischer & Klotz 1999) for plots with an approximate location for each plot. Quasi-permanent plots can be relocated using a plot-specific geographic position. We refer to ‘non-traceable’ plots if plot-specific location information is not available, i.e. plots can only be relocated to a physically and environmentally relatively homogeneous area. The discrimination between permanent, quasi-permanent, and non-traceable plots has important implications for the methods we can use to analyse vegetation change as we discuss below.
Fig. 1.
Types of plots available for resurveys: (a) permanent, (b) quasi-permanent, and (c) non-traceable plots. Filled symbols denote the plot centers’ locations in the initial survey, outlined symbols denote the plot centers’ locations in the resurvey. Whereas the position of permanently marked plots is known exactly, the location variability is increasing from quasi- to non-traceable plots. For non-traceable plots, plot locations are not known for individual plots (their positions are drawn here to illustrate the case) but for groups of plots only, i.e. a certain number of plots is known to have been sampled in a specific area. Therefore, one-to-one comparisons are not possible. Figure is adopted from Chytrý et al. 2014.
Challenges in resurveying historical vegetation data
Relocation error
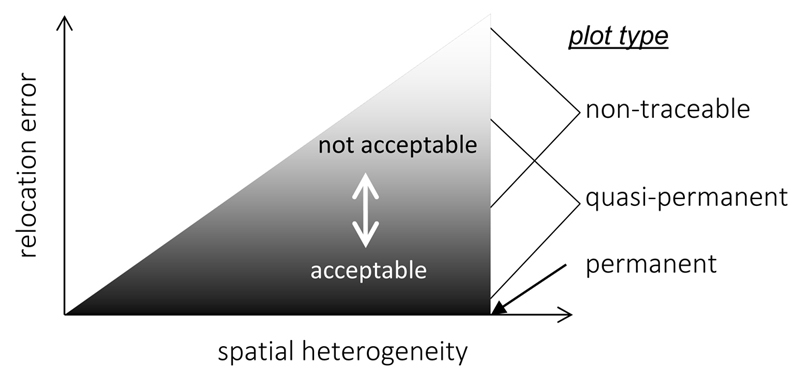
Location imprecision of historical plots will cause a mismatch in the position of historical and resurveyed plots and therefore has been a concern of researchers for at least two decades (Fischer & Stöcklin 1997; Hédl 2004; Bennie et al. 2006; Ross et al. 2010; Kopecký & Macek 2015). Problems in relocation of historical plots may cause pseudo-turnover which adds a random error to the temporal change in vegetation making it difficult to detect the real temporal trend and deem it statistically significant in a test (i.e. increase the Type II error). The errors caused by plot relocation may change from negligible for permanent plots to significant for quasi-permanent or non-traceable plots (Fig. 2). The added variability caused by relocation mismatch also depends on the spatial heterogeneity of the vegetation under study. Under the – unlikely – condition of perfectly homogeneous vegetation, relocation imprecision would not add variability to the result of the analysis of temporal change. In this case plots could be resurveyed in a completely random spatial pattern (using non-traceable plots) which would provide the same result as a resurvey of permanent plots. In the real world, vegetation always has a certain degree of spatial heterogeneity and the higher it is (the finer the “grain” of vegetation mosaics), the larger the variability caused by relocation error can be expected (Fig. 2). As long as the relocation error is not causing bias, i.e. a directed deviation in vegetation composition, the variability in the estimated temporal change caused by relocation imprecision can be reduced by increasing the sample size.
Fig. 2.
Relocation error increases with spatial heterogeneity of vegetation. Permanent plots have theoretically zero relocation error, while it gradually increases as plot relocation moves from approximate (as in quasi-permanent plots) to random (non-traceable plots). Note that there is no sharp boundary between the two latter plot types. The decision on whether the relocation error is acceptable or not is largely arbitrary and can have relatively broad range depending on spatial heterogeneity and quality of plot relocation.
With increasing distance between the historical and the resampled plots, the variability in estimates of change in species composition and diversity will increase to an extent reaching its potential maximum for non-traceable plots. The distinction between quasi-permanent and non-traceable plots is pragmatic and cannot be directly translated as to the reliability of the results of a plot resurvey. The reliability of a resurvey study depends on vegetation heterogeneity and an arbitrary decision of an acceptable degree of relocation error (Fig. 2). The effects of plot relocation imprecision may be minor in comparison with temporal change for quasi-permanent plots and compositional turnover estimates may be quite robust if efforts are made to keep the resampling error low (Hédl 2004; Ross et al. 2010; Chytrý et al. 2014; Kopecký & Macek 2015). However, plot relocation error is unavoidable for non-traceable plots and cannot be avoided completely for quasi-permanent plots. For the latter, relocation error can (and should) be reduced by locating plots as close as possible to their original locations through considering all information available from the original study (e.g., maps, descriptions of plot positions, elevation, aspect, slope, photographs). In addition to reducing relocation error when using quasi- and non-traceable plots, photographs of permanent plots and of study sites may be useful tools to support the documentation of vegetation change and should be considered routine for any kind of survey and resurvey.
Observer bias
In most cases resurveys of historical plots are conducted by persons other than the original surveyors. At the same time, more than one person is sometimes involved in either sampling or resampling. Variability due to multiple observers may result in an observer bias, which refers to observer-related differences in composition (identification bias) and quantitative properties (abundance bias) among vegetation samples (Scott & Hallam 2002; Archaux et al. 2006; Vittoz & Guisan 2007; Burg et al. 2015). The observer bias is independent of whether permanent, quasi-permanent, or non-traceable plots are used. In theory, it is also independent from the effects of seasonality discussed below. Although observer bias is completely independent from relocation error, both can increase with the spatial heterogeneity of the vegetation. Temporal patterns in species richness may be biased depending on the number of investigators conducting the sampling (Archaux et al. 2006; Burg et al. 2015). Although species richness is expected to increase with increased sampling effort, or with the skill of the observer, this will, in itself, not cause a bias for quantification of differences in species composition as long as there is no bias owing to species identification or taxonomy. The observer bias is difficult to avoid and it is also difficult to control for it when comparing two surveys. One way of assessing the potential effect of identification bias is to have a look at the species that have changed most, and assess if these species are particularly difficult to identify, if they can easily be confused with another species, or if their taxonomy has changed over time. If the large changes are found in species that are both easy to detect and to identify the changes are less likely to be due to observer biases.
Seasonality and other sources of temporal variability
Most datasets resulting from vegetation resampling consist of data representing vegetation communities at two different points in time. The lack of a time series following the vegetation’s development over time and in response to environmental variation may be another source of uncertainty in resurvey studies. For instance, it is not clear how much of the observed change would be due to the phenological stage of the vegetation as it may be caused by temporal or spatial variation in, for example, precipitation regimes (e.g. Cleland et al. 2013). Moreover, the particular timing of a resurvey may influence the results if the resampling is conducted at a different phenological date than the original sampling, potentially resulting in over- or under-estimation of species abundance (Vymazalová et al. 2012). If past weather data are available, phenological periods may be deducted from temperature data (for instance through growing degree days) enabling to resurvey plots in a phenological period comparable to the historical survey.
Methods for analysing vegetation change
Analysing vegetation change with permanent plot data
In the case of permanent plots, the analysis is usually straightforward and vegetation changes can be tested statistically along any gradient. To study community changes, ordination methods (Legendre & Legendre 2012) are commonly used, where a change in species composition over time can be estimated along the ordination axes (e.g. Kopecký et al. 2013; Chytry et al. 2014). This can be done either by a paired t-test on sample scores from an indirect ordination or by a restricted permutation test using a direct ordination. The environmental drivers behind the changes may be identified by fitting environmental variables along the ordination axes that are found to correspond to changes over time, or the changes can be related to the differences in sample scores between two time periods. Bias in species identifications between time periods may identify artificial community shifts, but apart from this, the observed difference in communities can be regarded as true community shifts.
Analysing vegetation change with quasi-permanent plot data
For quasi-permanent plots, the same methods as for permanent plots may be adequate as long as relocation imprecision is low (Fig. 1), i.e. we can still compare one plot in the initial survey with its counterpart in the resurvey in a paired t-test manner. When interpreting the results, however, we need to remember that uncertainty not only derives from observer bias and natural inter-annual variability but also relocation error (Fig. 2). The error added to temporal change may become high and conflate the real change. To gain information about both the real change and the relocation error Ross et al. (2010) suggest replicating plots in the resurvey (e.g. 3 to 5 replicates per original plot) as close to the best estimate of the original plots as possible. This over-sampling enables the comparison of species turnover (as estimated from the original and the resurvey’s replicate plots) with the pseudo-turnover (as estimated by comparing the replicate plots) and allows the real change to be corrected for the error caused by spatial pseudo-turnover. Ross et al. (2010) further suggest that, if relocation variability is small compared to the change in time, we may safely use the analysis to investigate changes over time. However, it should be emphasised that relocation imprecision will result in higher variability and a lower chance of finding statistically significant changes when such a change is present (Type II error), and that a larger sample (this depends also on the historical dataset) will increase the power to detect real changes as it will decrease the variability of the mean of the two surveys.
Analysing vegetation change with non-traceable plot data
Non-traceable plot data are different from permanent and quasi-permanent plot data because the plots in the original survey and the resurvey cannot be compared one to one (Fig. 1). Before data analysis, specific additional steps in the data preparation are needed. For instance, if numbers of plots differ between the historical sampling and the resampling because not all plots were resurveyed and because those resampled cannot be paired, the datasets would require standardisation before calculating and testing for a temporal change. This can be done by relating the total number of species to the total number of plots in each survey, or species’ frequencies of occurrence may be standardised with the total number of occurrences of a particular species. Ordination techniques may be appropriate to prepare data when dealing with random sampling in a restricted area to detect and remove outliers indicating that sampling was conducted in dissimilar plant communities (e.g. Felde et al. 2012).
To enable the analysis of temporal change with non-traceable plot data, we have to adapt our methodology. First of all, we need to make several assumptions, and in some cases it is impossible to separate a sampling bias from a community change. In cases where we can assume that the survey and resurvey data have been recorded randomly in a restricted area, the old and new datasets can still be compared with a t-test, but no longer with a paired t-test. However, since the original data were often collected for phytosociological purposes, a random distribution of the plots along environmental gradients is rather unlikely and cannot be assumed. When environmental data are missing from the historical studies it is difficult to account for the differences in numerical analyses to disentangle real changes in vegetation or environment from changes caused by pseudo-turnover (Chytrý et al. 2014).
Analysing vegetation change along environmental gradients with non-traceable plots
If the historical sampling is done along a geographical gradient, resurveying can be done along the same gradient, and different approaches may be used to quantify the changes even though non-traceable plots are used. Comparable grouping along environmental gradients may be a key step in data preparation for comparisons of plant communities over time, particularly when dealing with unequal numbers of plots (Kapfer et al. 2011) or when environmental data are not explicit (e.g. location within an elevational belt; Felde et al. 2012). Lenoir et al. (2008) used logistic regression to calculate species optima along an elevational gradient and compared these optima for the same species between two time periods. Felde et al. (2012) followed a similar approach to assess how species optima had changed along an elevational gradient. Bertrand et al. (2011) took an innovative approach commonly applied in palaeoecological studies - weighted averaging partial least squares (ter Braak & van Dam 1989). They used 75,000 plant assemblages sampled over 44 years, and each of these assemblages had an observed associated temperature. A subset of these assemblages was used as a training dataset to establish a relationship (a transfer or calibration function) between the assemblages and the observed temperature. From this they could then predict the temperature in the remaining plots based on the floristic assemblages, which in turn was compared with the observed temperature. When the observed temperature was warmer than the predicted temperature from floristic assemblages it was interpreted as communities lagging behind the climatic warming in that area. Because they compared the predicted and observed temperature from the same plot with the same geographic location they omitted any problems related to relocation of plots.
Other studies have calculated weighted averages of species indicator values (e.g. Ellenberg et al. 1992; Landolt et al. 2010) to represent plot specific environmental factors (e.g. soil pH, nutrients) in order to reconstruct environmental gradients on the basis of vegetation compositional data (e.g. Thimonier et al. 1994; Diekmann & Dupre 1997; Keith et al. 2009; Hédl et al. 2010; Kapfer et al. 2011). Changes observed from comparing the average indicator values of the historic dataset and the resurvey will suggest that the species composition has changed for the particular environmental gradient considered, but this assumes that the sampling in both time periods was random; if this is not the case, the observed changes in any average indicator value might be caused by sampling bias. Accounting for non-random sampling will often mean that any environmental changes have to be removed and make a direct comparison impossible. However, as the species react individualistically to environmental changes, species may change internally in the communities. Such changes may be detectable even after accounting for potential sampling biases (Kapfer et al. 2011).
Conclusions
This paper provides a brief account on resurveying historical plot-based vegetation data. Resurveying of historical vegetation data can provide unique insights into vegetation changes in relation to environmental change over decades, and despite some challenges in using such data, the wealth of historical plot data available represents a valuable source for understanding long-term vegetation dynamics and how vegetation responds to different drivers.
We propose the following recommendations when using non-permanently marked historical plots to study vegetation dynamics:
The historical vegetation plots considered for resampling should be equipped with information of at least approximate plot location within a study area (e.g. topography, position on maps, management compartment, photographs, etc.).
Before resampling, plots should be relocated as precisely as possible by considering all information available from the historical resource.
Resampling should be conducted in the period of the year phenologically similar or comparable to the historical survey. This will control for seasonal variability of vegetation.
Field workers for the resurvey should be well trained field botanists in order to keep observer bias acceptable.
The data from survey and resurvey need to be standardised (nomenclature, number of plots) and appropriate statistical tools should be used, taking into account the sampling protocols of the different (re)surveys.
After all for any vegetation resampling, it is a prerequisite to match the methods that have been used in the original study as close as possible for reasons of comparability. If important information (see points 1-3 above) is not available, the conduction of a resurvey should be reconsidered since uncertainties may be too high for a meaningful analysis of change over time.
Acknowledgements
The research leading to these results has received funding from the European Research Council under the European Union's Seventh Framework Programme (FP7/2007–2013)/ERC Grant agreement no 278065. The research leading to these results has received funding from the Polish-Norwegian Research Programme operated by the National Centre for Research and Development under the Norwegian Financial Mechanism 2009-2014 in the frame of Project Contract No. Pol-Nor/196829/87/2013. Additional funding support was received by The Fram Centre (project no.: 362202).
References
- Archaux F, Gosselin F, Berg L, Chevalier R. Effects of sampling time, species richness and observer on the exhaustiveness of plant censuses. Journal of Vegetation Science. 2006;17:299–306. [Google Scholar]
- Bakker JP, Olff H, Willems JH, Zobel M. Why do we need permanent plots in the study of long-term vegetation dynamics? Journal of Vegetation Science. 1996;7:147–156. [Google Scholar]
- Bernhardt-Römermann M, Baeten L, Craven D, De Frenne P, Hédl R, Lenoir J, Bert D, Brunet J, Chudomelová M, Decocq G, Dierschke H, et al. Drivers of temporal changes in temperate forest plant diversity vary across spatial scales. Global Change Biology. 2015;21:3726–3737. doi: 10.1111/gcb.12993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand R, Lenoir J, Piedallu C, Riofrío-Dillon G, de Ruffray P, Vidal C, Pierrat J-C, Gégout J-C. Changes in plant community composition lag behind climate warming in lowland forests. Nature. 2011;479:517–520. doi: 10.1038/nature10548. [DOI] [PubMed] [Google Scholar]
- Bobbink R, Hicks K, Galloway J, Spranger T, Alkemade R, Ashmore M, Bustamante M, Cinderby S, Davidson E, Dentener F, Emmett B, et al. Global assessment of nitrogen-deposition effects on terrestrial plant diversity: a synthesis. Ecological Applications. 2010;20(1):30–59. doi: 10.1890/08-1140.1. [DOI] [PubMed] [Google Scholar]
- Braun-Blanquet J. Pflanzensoziologie. Grundzüge der Vegetationskunde. Springer; Wien: 1964. [Google Scholar]
- Burg S, Rixen C, Stöckli V, Wipf S. Observation bias and its causes in botanical surveys on high-alpine summits. Journal of Vegetation Science. 2015;26:191–200. [Google Scholar]
- Bühler C, Roth T. Spread of common species results in local-scale floristic homogenization in grassland of Switzerland. Diversity and Distributions. 2011;17:1089–1098. [Google Scholar]
- Chapin FS, Shaver GR, Giblin AE, Nadelhoffer KJ, Laundre JA. Responses of arctic Tundra to experimental and observed changes in climate. Ecology. 1995;76(3):694–711. [Google Scholar]
- Chytrý M, Danihelka J. Long-term changes in the field layer of oak and oak-hornbeam forests under the impact of deer and mouflon. Folia Geobotanica et Phytotaxonomica. 1993;28:225–245. [Google Scholar]
- Chytrý M, Tichý L, Hennekens SM, Schaminée JHJ. Assessing vegetation change using vegetation-plot databases: a risky business. Applied Vegetation Science. 2014;17:32–41. [Google Scholar]
- Cleland EE, Collins SL, Dickson TL, Farrer EC, Gross KL, Gherardi LA, Hallett LM, Hobbs RJ, Hsu JS, Turnbull L, Suding KN. Sensitivity of grassland plant community composition to spatial vs. temporal variation in precipitation. Ecology. 2013;94:1687–1696. doi: 10.1890/12-1006.1. [DOI] [PubMed] [Google Scholar]
- Daniëls FJA, De Molenaar JG, Chytrý M, Tichý L. Vegetation change in Southeast Greenland? Tasiilaq revisited after 40 years. Applied Vegetation Science. 2011;14:230–241. [Google Scholar]
- Dengler J, Jansen F, Glöckler F, Peet RK, De Cáceres M, Chytrý M, Ewald J, Oldeland J, Lopez-Gonzalez G, Finckh M, Mucina L, et al. The Global Index of Vegetation-Plot Databases (GIVD): a new resource for vegetation science. Journal of Vegetation Science. 2011;22:582–597. [Google Scholar]
- Diekmann M, Dupre C. Acidification and eutrophication of deciduous forests in northwestern Germany demonstrated by indicator species analysis. Journal of Vegetation Science. 1997;8:855–864. [Google Scholar]
- Du Rietz GE. Zur methodologischen Grundlage der modernen Pflanzensoziologie. Dissertation, University of Uppsala; Uppsala: 1921. [Google Scholar]
- Ellenberg H, Weber HE, Düll R, Wirth V, Werner W, Paulissen D. Zeigerwerte von Pflanzen in Mitteleuropa. Scripta Geobotanica. 1992;18:1–258. [Google Scholar]
- Felde VA, Kapfer J, Grytnes J-A. Upward shift in elevational plant species ranges in Sikkilsdalen, central Norway. Ecography. 2012;35(10):922–932. [Google Scholar]
- Fischer M, Stöcklin J. Local extinctions of plants in remnants of extensively used calcareous grasslands 1950-1985. Conservation Biology. 1997;11(3):727–737. [Google Scholar]
- Fischer A. Floristical changes in Central European forest ecosystems during the past decades as an expression of changing site conditions. EFI-Proceedings. 1999;27:53–64. [Google Scholar]
- Fischer A, Klotz S. Zusammenstellung von Begriffen, die in der Vegetations-Dauerbeobachtung eine zentrale Rolle spielen. Tuexenia. 1999;19:10–12. [Google Scholar]
- Grabherr G, Gottfried M, Pauli H. Climate effects on mountain plants. Nature. 1994;369(6480):448. doi: 10.1038/369448a0. [DOI] [PubMed] [Google Scholar]
- Grytnes J-A, Kapfer J, Jurasinski G, Birks HH, Henriksen H, Klanderud K, Odland A, Ohlson M, Wipf S, Birks HJB. Identifying the driving factors behind observed elevational range shifts on European mountains. Global Ecology and Biogeography. 2014;23:876–884. [Google Scholar]
- Gunnarsson U, Rydin H, Sjors H. Diversity and pH changes after 50 years on the boreal mire Skattlössbergs Stormosse, Central Sweden. Journal of Vegetation Science. 2000;11:277–286. [Google Scholar]
- Harrison S, Damschen EI, Grace JB. Ecological contingency in the effects of climatic warming on forest herb communities. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:19362–19367. doi: 10.1073/pnas.1006823107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hédl R. Vegetation of beech forests in the Rychlebske Mountains, Czech Republic, re-inspected after 60 years with assessment of environmental changes. Plant Ecology. 2004;170:243–265. [Google Scholar]
- Hédl R, Kopecký M, Komárek J. Half a century of succession in a temperate oakwood: from species-rich community to mesic forest. Diversity and Distributions. 2010;16:267–276. [Google Scholar]
- Kapfer J, Grytnes J-A, Gunnarsson U, Birks HJB. Fine-scale changes in vegetation composition in a boreal mire over 50 years. Journal of Ecology. 2011;99:1179–1189. [Google Scholar]
- Keith Sa, Newton AC, Morecroft MD, Bealey CE, Bullock JM. Taxonomic homogenization of woodland plant communities over 70 years. Proceedings of the Royal Society B: Biological Sciences. 2009;276:3539–3544. doi: 10.1098/rspb.2009.0938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AE, Goulden ML. Rapid shifts in plant distribution with recent climate change. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:11823–22826. doi: 10.1073/pnas.0802891105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klanderud K, Birks HJB. Recent increases in species richness and shifts in altitudinal distributions of Norwegian mountain plants. The Holocene. 2003;13:1–6. [Google Scholar]
- Koch M, Jurasinski G. Four decades of vegetation development in a percolation mire complex following intensive drainage and abandonment. Plant Ecology & Diversity. 2015;8:49–60. [Google Scholar]
- Kopecký M, Hédl R, Szabó P. Non-random extinctions dominate plant community changes in abandoned coppices. Journal of Applied Ecology. 2013;50:79–87. doi: 10.1111/1365-2664.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecký M, Macek M. Vegetation resurvey is robust to plot location uncertainty. Diversity and Distributions. 2015;21:322–330. doi: 10.1111/ddi.12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landolt E, Bäumler B, Erhardt A, Hegg O, Klötzli F, Lämmler W, Nobis M, Rudmann-Maurer K, Schweingruber FH, Theurillat J-P, Urmi E, et al. Flora Indicativa – Ökologische Zeigerwerte und biologische Kennzeichen zur Flora der Schweiz und der Alpen. Haupt; Bern, Stuttgart, Wien: 2010. [Google Scholar]
- Lawesson JE. A concept for vegetation studies and monitoring in the Nordic Countries. Nordic Council of Ministers; Copenhagen: 2000. [Google Scholar]
- Lenoir J, Gégout JC, Marquet PA, Ruffray P, Brisse H. A significant upward shift in plant species optimum elevation during the 20th century. Science. 2008;320:1768–1771. doi: 10.1126/science.1156831. [DOI] [PubMed] [Google Scholar]
- Pauli H, Gottfried M, Dullinger S, Abdaladze O, Akhalkatsi M, Alonso JLB, Coldea G, Dick J, Erschbamer B, Fern B, Calzado R, et al. Recent plant diversity changes on Europe’s mountain summits. Science. 2012;336:353–355. doi: 10.1126/science.1219033. [DOI] [PubMed] [Google Scholar]
- Persson S. Succession in a south Swedish deciduous wood: a numerical approach. Vegetatio. 1980;43:103–122. [Google Scholar]
- Ross LC, Woodin SJ, Hester A, Thompson DBA, Birks HJB. How important is plot relocation accuracy when interpreting re-visitation studies of vegetation change? Plant Ecology & Diversity. 2010;3:1–8. [Google Scholar]
- Schaminée JHJ, Hennekens SM, Chytrý M, Rodwell JS. Vegetation-plot data and databases in Europe: an overview. Preslia. 2009;81:173–185. [Google Scholar]
- Scott WA, Hallam CJ. Assessing species misidentification rates through quality assurance of vegetation monitoring. Plant Ecology. 2002;165:101–115. [Google Scholar]
- Šebesta J, Šamonil P, Lacina J, Oulehle F, Houška J, Buček A. Acidification of primeval forests in the Ukraine Carpathians: vegetation and soil changes over six decades. Forest Ecology and Management. 2011;262:1265–1279. [Google Scholar]
- Silvertown J, Poulton P, Johnston E, Edwards G, Heard M, Biss PM. The Park Grass experiment 1856-2006: its contribution to ecology. Journal of Ecology. 2006;94:801–814. [Google Scholar]
- Tape K, Sturm M, Racine C. The evidence for shrub expansion in Northern Alaska and the Pan-Arctic. Global Change Biology. 2006;12:686–702. [Google Scholar]
- Ter Braak CJF, van Dam H. Inferring pH from diatoms: a comparison of old and new calibration methods. Hydrobiologia. 1989;178:209–223. [Google Scholar]
- Thimonier A, Dupouey JL, Bost F, Becker M. Simultaneous eutrophication and acidification of a forest ecosystem in North-East France. New Phytologist. 1994;126:533–539. doi: 10.1111/j.1469-8137.1994.tb04252.x. [DOI] [PubMed] [Google Scholar]
- Van Calster H, Baeten L, Verheyen K, de Keersmaeker L, Dekeyser S, Rogister JE, Hermy M. Diverging effects of overstorey conversion scenarios on the understorey vegetation in a former coppice-with-standards forest. Forest Ecology and Management. 2008;256:519–528. [Google Scholar]
- Van den Berg LJL, Vergeer P, Rich TCG, Smart SM, Guest D, Ashmore MR. Direct and indirect effects of nitrogen deposition on species composition change in calcareous grasslands. Global Change Biology. 2011;17:1871–1883. [Google Scholar]
- Vellend M, Baeten L, Myers-Smith IH, Elmendorf SC, Beauséjour R, Brown CD, de Frenne P, Verheyen K, Wipf S. Global meta-analysis reveals no net change in local-scale plant biodiversity over time. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:19456–19459. doi: 10.1073/pnas.1312779110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheyen K, Baeten L, De Frenne P, Bernhardt-Römermann M, Brunet J, Cornelis J, Decocq G, Dierschke H, Eriksson O, Hédl R, Heinken T, et al. Driving factors behind the eutrophication signal in understorey plant communities of deciduous temperate forests. Journal of Ecology. 2012;100:352–365. [Google Scholar]
- Vittoz P, Guisan A. How reliable is the monitoring of permanent vegetation plots? A test with multiple observers. Journal of Vegetation Science. 2007;18:413–422. [Google Scholar]
- Vymazalová M, Axmanová I, Tichý L. Effect of intra-seasonal variability on vegetation data. Journal of Vegetation Science. 2012;23:978–984. [Google Scholar]
- Waller DM, Amatangelo KL, Johnson S, Rogers DA. Wisconsin Vegetation Database – plant community survey and resurvey data from the Wisconsin Plant Ecology Laboratory. Biodiversity & Ecology. 2012;4:255–264. [Google Scholar]
- Walther G-R, Berge S, Sykes MT. An ecological ‘footprint’ of climate change. Proceedings of the Royal Society. 2005;272:1427–1432. doi: 10.1098/rspb.2005.3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis KJ, Birks HJB. What is natural? The need for a long-term perspective in biodiversity conservation. Science. 2006;314:1261–1265. doi: 10.1126/science.1122667. [DOI] [PubMed] [Google Scholar]
- Wilson SD, Nilsson C. Arctic alpine vegetation change over 20 years. Global Change Biology. 2009;15:1676–1684. [Google Scholar]
- Wipf S, Stöckli V, Herz K, Rixen C. The oldest monitoring site of the Alps revisited: accelerated increase in plant species richness on Piz Linard summit since 1835. Plant Ecology & Diversity. 2013;6:447–455. [Google Scholar]