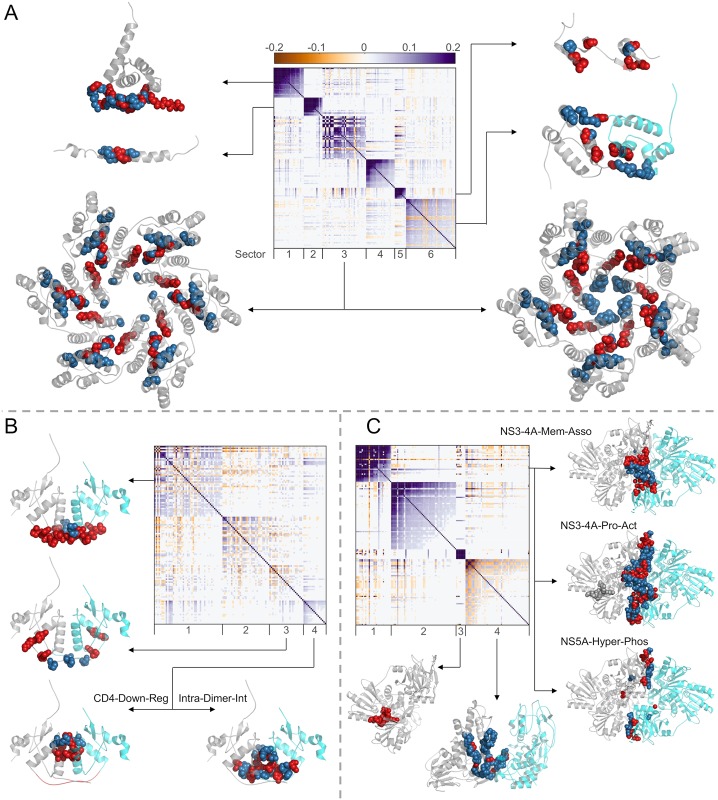
Fig 6. Details of the different biochemical domains of viral proteins associated with the respective inferred RoCA sectors.
Sectors are shown as diagonal blocks in the heat map of cleaned correlations with rows and columns re-ordered accordingly (Materials and methods); the heat map is restricted to sector residues only. For each sector, the crystal structure of the associated domains are depicted, when available. The protein chains are shown in gray (in case of dimer structures, chains A and B are depicted in gray and cyan colors, respectively), the relevant domain residues present in the sector are represented as red spheres, and the remaining domain residues are shown as blue spheres. (A) For HIV Gag, sector 1 residues are associated with the membrane-binding domain of p17 (PDB ID 2LYA); sector 2 residues with the p24-SP1 interface (PDB ID 1U57); sector 3 residues with the intra-hexamer and intra-pentamer interface of p24 (PDB ID 3GV2 and 3P05, respectively); sector 5 residues with the two zinc-finger structures of p7 (PDB ID 1MFS); and sector 6 residues with the inter-hexamer interface of p24 (PDB ID 2KOD). No crystal structure is available for the SP2-p6 interface residues that comprise sector 4. (B) For HIV Nef, relevant residues of the biochemical domains are shown on the dimer crystal structure (PDB ID 4U5W). Note that this crystal structure only includes residues 68-204 of Nef. Sector 1 residues are associated with the viral infectivity enhancement function; sector 3 residues with HLA1 down-regulation function (note that four residues (62-65) of this biochemical domain cannot be shown in this crystal structure); and sector 4 residues with both the CD4 down-regulation function and the intra-dimer interface (important for Nef dimerization). (C) For HCV NS3-4A, sector 1 residues are associated with the interface between NS3 and NS4A proteins (PDB ID 4B6E; this crystal structure only includes NS4A residues 21-36) important for (i) membrane association and assembly of a functional HCV replication complex, (ii) activation of the NS3 protease function, and (iii) NS5A hyper-phosphorylation; sector 3 residues with the motif critical for enzymatic and helicase activities of NS3 (no blue sphere is visible as sector 3 comprises all the residues in this motif); and sector 4 residues with the helicase-helicase interface of the NS3 dimer (PDB ID 2F55).