Key Points
Question
What was the cause of uveal effusion in 3 patients taking immune checkpoint inhibitors?
Findings
In this case series, uveal effusion developed in 3 patients who had been recently taking systemic antiprogrammed cell death protein-1 and antiprogrammed cell death ligand-1 monoclonal antibody therapy.
Meaning
Immune checkpoint inhibitors, including antiprogrammed cell death protein-1 and antiprogrammed cell death ligand-1 monoclonal antibodies, may cause uveal effusion.
This article describes 3 patients who developed uveal effusion after taking systemic antiprogrammed cell death protein-1 and ligand-1 monoclonal antibody therapy.
Abstract
Importance
Immune checkpoint inhibitors, including antiprogrammed cell death protein-1 (anti-PD-1) and antiprogrammed cell death ligand-1 (anti-PD-L1) monoclonal antibodies, have recently been introduced as a promising new immunotherapy for solid cancers. The adverse effects typically include inflammation of the skin, endocrine, and gastrointestinal systems.
Objective
To describe 3 patients who developed uveal effusion after initiating anti-PD-1 and anti-PD-L1 monoclonal antibody therapy.
Design, Setting, and Participants
This case series was conducted in a university-based ocular oncology practice. The participants were a 68-year-old African American man with metastatic adenocarcinoma of the lung and 2 white men, aged 52 years and 85 years, with metastatic cutaneous melanoma; all were taking anti-PD-1 and anti-PD-L1 monoclonal antibody therapy.
Main Outcomes and Measures
Ocular findings of 3 patients.
Results
We identified 3 patients who developed uveal effusion within 1 to 2 months after initiating anti-PD-1 and anti-PD-L1 monoclonal antibody therapy. Uveal effusion resolved completely in 6 to 12 weeks after discontinuation of systemic therapy in 2 patients and persisted in 1 patient who continued the therapy.
Conclusions and Relevance
Uveal effusion should be considered in patients taking anti-PD-1 and/or PD-L1 monoclonal antibody therapy. Because of the role of the PD-1 pathway in the inhibition of self-reactive T cells, PD-1 inhibition might lead to inflammation because of immune-related adverse effects.
Introduction
Uveal effusion may be idiopathic or might develop from trauma, drugs, or inflammatory conditions. Immune checkpoint inhibitors, including antiprogrammed cell death protein-1 (anti-PD-1) and antiprogrammed cell death ligand-1 (anti-PD-L1) monoclonal antibodies have emerged as promising immunotherapy for solid cancers. Adverse reactions to these medications typically manifest as immune-related events, and ocular adverse effects are rare.1,2 However, we present 3 cases of uveal effusion after immune checkpoint inhibitor therapy.
Case Series
Case 1
A 68-year-old African American man presented with blurred vision and redness in his left eye that had worsened during the previous 2 weeks. He had lung adenocarcinoma with adrenal metastases and had received 2 infusions of atezolizumab (an anti-PD-L1 monoclonal antibody); the first infusion was 1 month prior to presentation, and the second was 3 days prior to presentation.
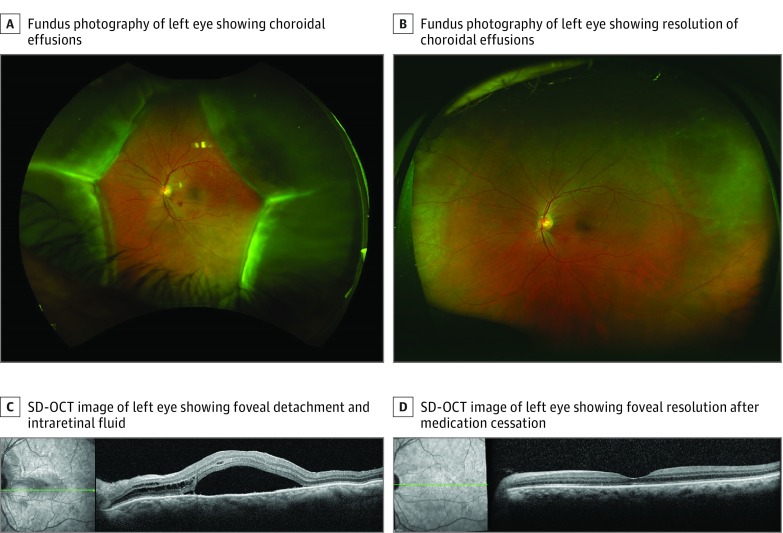
At presentation, his visual acuity was 20/25 OD and 20/150 OS. Anterior segment examination of the left eye revealed mild conjunctival hyperemia, diffuse fine corneal endothelial pigment, and a 1+ grade of cells (defined as 6-15 cells in a field the size of 1 x 1 mm slit beam) in the anterior chamber. Fundus examination of the left eye showed a macula-involving exudative retinal detachment and a retinal hemorrhage in the inferior macula. There was a cotton wool spot superior to the optic disc and bullous serous choroidal detachment in all 4 quadrants (Figure 1A).
Figure 1. Images From Patient 1 at Presentation and After Cessation of Antiprogrammed Cell Death Protein-1/Antiprogrammed Cell Death Ligand-1 (Anti-PD-1/Anti-PD-L1) Therapy.
A and C, Images from patient 1 at initial presentation; B and D, Images from patient 1 after cessation of anti-PD-1/PD-L1 therapy. A, Fundus photography of the left eye showing choroidal effusions in all 4 quadrants; SD-OCT indicates spectral-domain optical coherence tomography.
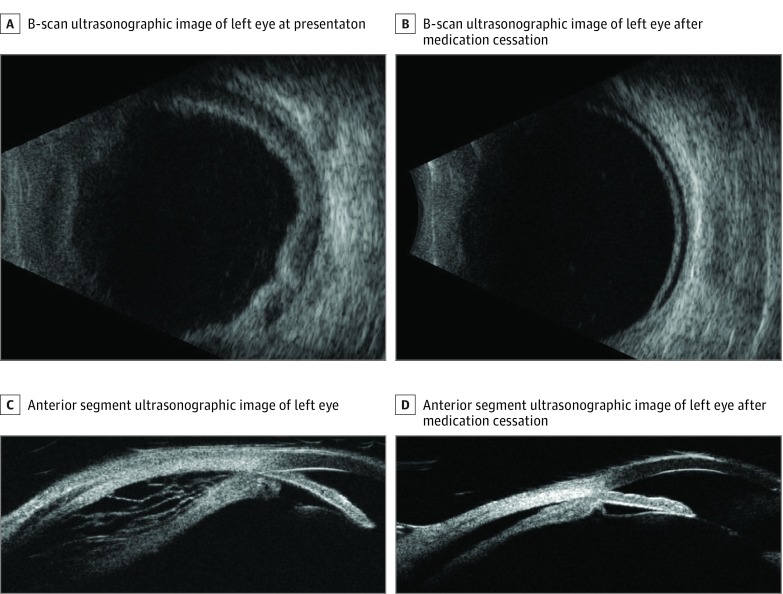
A B-scan ultrasonographic image of the left eye showed a 360-degree serous choroidal detachment with a shallow exudative retinal detachment. No intraocular masses were detected (eFigure 1A in the Supplement). On ultrasound biomicroscopy of the anterior segment, the anterior chamber was shallow with a closed angle (eFigure 1C in the Supplement). Spectral-domain optical coherence tomography confirmed the presence of subretinal and intraretinal fluid involving the fovea (Figure 1C).
His systemic therapy was discontinued. Three months later, his vision in the left eye improved to 20/40, and ciliochoroidal effusions and subretinal fluid had completely resolved (Figure 1B and D and eFigure 1B and D in the Supplement).
Case 2
A 52-year-old white man presented with redness and pain in both eyes for 3 weeks. He had cutaneous melanoma with metastases to axillary lymph nodes. Nivolumab (an anti-PD-1 monoclonal antibody) administration had begun 3 weeks prior to presentation, and he had received 2 infusions. His second infusion had occurred 1 week prior to presentation.
On presentation, his visual acuity was 20/100 OD and 20/40 OS. Intraocular pressures were 38 mm Hg OD and 53 mm Hg OS. Anterior segment examination of both eyes showed conjunctival hyperemia with shallow anterior chambers, with a 2+ grade of cells (defined as 16-25 cells in in a field the size of 1 x 1 mm slit beam) OD and 1+ grade of cells OS, and mild cataracts (eFigure 2A and B in the Supplement). Anterior chamber angles were closed. Fundus examination of both eyes revealed a 360-degree annular serous choroidal detachment with a few dot-blot hemorrhages in the peripheral retina. Ultrasound biomicroscope and B-scan ultrasonography results confirmed these findings. No intraocular masses were detected (Figure 2A and C and eFigure 3A and C in the Supplement). The patient was treated with peripheral iridotomies and topical brimonidine, dorzolamide, timolol, prednisolone acetate, and atropine in both eyes.
Figure 2. Images From Patient 2 at Presentation and After Cessation of Antiprogrammed Cell Death Protein-1/Antiprogrammed Cell Death Ligand-1 (Anti-PD-1/Anti-PD-L1) Therapy.
Note choroidal effusions (A and C) that resolve after agent use cessation (B and D).
A week later, choroidal effusions progressed in both eyes, with deterioration of his vision to 20/100 and 20/250. His systemic immunotherapy was discontinued. Six weeks later, his vision improved to 20/60 OD and 20/30 OS, and intraocular pressures decreased to 15 mm Hg OD and 25 mm Hg OS. On slitlamp examination, anterior chambers had deepened in both eyes. On B-scan ultrasonography results, choroidal detachments improved in both eyes (Figure 2B and D; and eFigure 3B and D in the Supplement).
Case 3
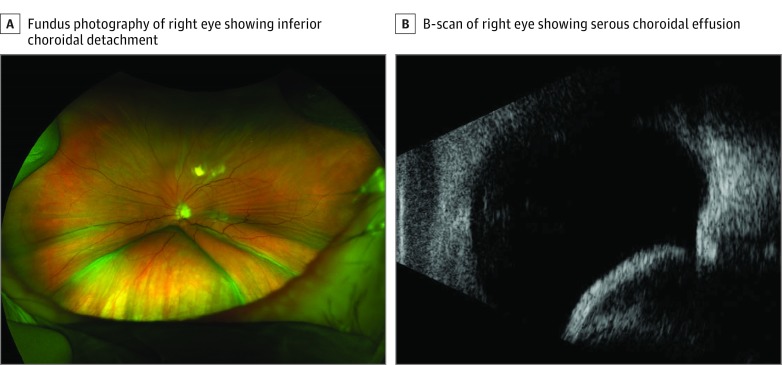
A white man aged 85 years presented with periorbital swelling of the left eye. He had desmoplastic melanoma of his left cheek treated with multiple surgical procedures and external beam radiotherapy. Pembrolizumab (anti-PD-1 monoclonal antibody) administration had begun 2 months prior because of recurrence, and his third infusion was 3 days before presentation.
On presentation, his vision was 20/20 OD and 20/200 OS. Anterior segment examination of the left eye showed mild edema of the eyelids and conjunctival chemosis without any cell or flare. Fundus examination of the left eye revealed macular striae and a serous choroidal detachment involving the inferior macula. (Figure 3A). A B-scan ultrasonographic image showed a bullous choroidal detachment inferiorly (Figure 3B). The recurrent melanoma was refractory to all treatment other than pembrolizumab, and the patient was not interested in undergoing surgical debulking. Therefore, after discussion with the patient’s oncologist, the patient decided to continue pembrolizumab despite his ocular issues. The tumor progressed, and the patient died 4 months after his initial presentation.
Figure 3. Images From Patient 3 at Presentation.
Discussion
Immune checkpoint proteins inhibit immune system function through various mechanisms; PD-1 is a receptor expressed on the surface of activated T cells, activated natural killer cells, B cells, and tumor-infiltrating lymphocytes. Engagement of T-cell PD-1 with PD-L1, its ligand, on the tumor cell surface inhibits the activation of T cells, thus allowing cancer cells to avoid immune surveillance.2,3 Conversely, the inhibition of this pathway augments the antitumor immune response. However, given that this mechanism is also responsible for maintaining beneficial T-cell tolerance to self-antigens in various organ systems, its inhibition can cause immune-related adverse effects.
Adverse effects of immune checkpoint inhibitors have been investigated in multiple studies. In a review4 of 576 patients with advanced melanoma who were treated with nivolumab, 71% of patients experienced treatment-related adverse effects. The most common adverse effects were fatigue (25%) and pruritus (17%). Clinically significant immune-related adverse effects occurred in 49% of patients; most commonly these were observed in skin (34%), gastrointestinal (13%), and endocrine (8%) systems. These are tissues that are under constant surveillance by the immune system, and thus the adverse events can resemble an autoimmune process. Immune-related adverse effects typically occurred after more than 1 infusion, and median onset ranged from 5 weeks to 15 weeks.4 One explanation may be that adverse effects manifest after a threshold immune response is met with multiple treatments. Consistent with these previous reports, the patients in this series presented between 3 and 8 weeks after starting anti-PD-1/PD-L1 therapy, with each patient receiving at least 2 infusions.
Ocular toxicities after the use of immune checkpoint inhibitors are uncommon. In a systematic review,2 uveitis and dry eye were the only ocular toxicities in patients taking PD-1/PD-L1 inhibitors. The incidence of uveitis ranged from 0.3% to 0.6%, and there was no information about the pattern, time course, or management of the uveitis. Other reported rare ocular adverse effects included retinopathy, clinical myasthenia gravis, and orbital inflammation.2 In 2 of the 3 cases in this series (67%), anterior chamber inflammation was noted. Although the presence of intraocular inflammation can be a cause of uveal effusion, the uveal effusions resolved after the systemic immunotherapy was discontinued, suggesting that the immunotherapy had a role in the causative mechanism of the uveal effusions in these patients.
We hypothesize that ocular toxicity from immune checkpoint inhibitor therapy stems from reprogramming of the cell death pathway in an immune privileged tissue. In support of this hypothesis, one study described5 a case of corneal graft rejection in a patient treated with nivolumab for primary lung cancer. Blockage of regulatory T-cell propagation was sufficient to promote corneal graft rejection in a rodent model. High levels of PD-L1 are found in many ocular tissues, and may be key to preventing autoimmunity.5 Blockade of such regulatory T cells may have triggered the toxicity seen in these 3 patients.
In summary, we present a case series of 3 patients who developed uveal effusion following treatment with immune checkpoint inhibitors. We recommend that ocular toxicity, including uveal effusion as described in these cases, be considered by both ophthalmologists and oncologists in the evaluation of patients taking anti-PD-1/PD-L1 agents.
eFigure 1. Images from patient #1 at initial presentation (A, C) and following cessation (B, D) of anti-PD-1/PD-L1 therapy.
eFigure 2. Images from patient #2.
eFigure 3. Images from patient #2 at presentation (A, C) and after cessation of anti-PD-1/PD-L1 therapy (B, D).
References
- 1.Elagouz M, Stanescu-Segall D, Jackson TL. Uveal effusion syndrome. Surv Ophthalmol. 2010;55(2):134-145. [DOI] [PubMed] [Google Scholar]
- 2.Abdel-Rahman O, Oweira H, Petrausch U, et al. . Immune-related ocular toxicities in solid tumor patients treated with immune checkpoint inhibitors: a systematic review. Expert Rev Anticancer Ther. 2017;17(4):387-394. [DOI] [PubMed] [Google Scholar]
- 3.Dong H, Strome SE, Salomao DR, et al. . Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8(8):793-800. [DOI] [PubMed] [Google Scholar]
- 4.Weber JS, Hodi FS, Wolchok JD, et al. . Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J Clin Oncol. 2017;35(7):785-792. [DOI] [PubMed] [Google Scholar]
- 5.Le Fournis S, Gohier P, Urban T, Jeanfaivre T, Hureaux J. Corneal graft rejection in a patient treated with nivolumab for primary lung cancer. Lung Cancer. 2016;102:28-29. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Images from patient #1 at initial presentation (A, C) and following cessation (B, D) of anti-PD-1/PD-L1 therapy.
eFigure 2. Images from patient #2.
eFigure 3. Images from patient #2 at presentation (A, C) and after cessation of anti-PD-1/PD-L1 therapy (B, D).