Fig. 14.
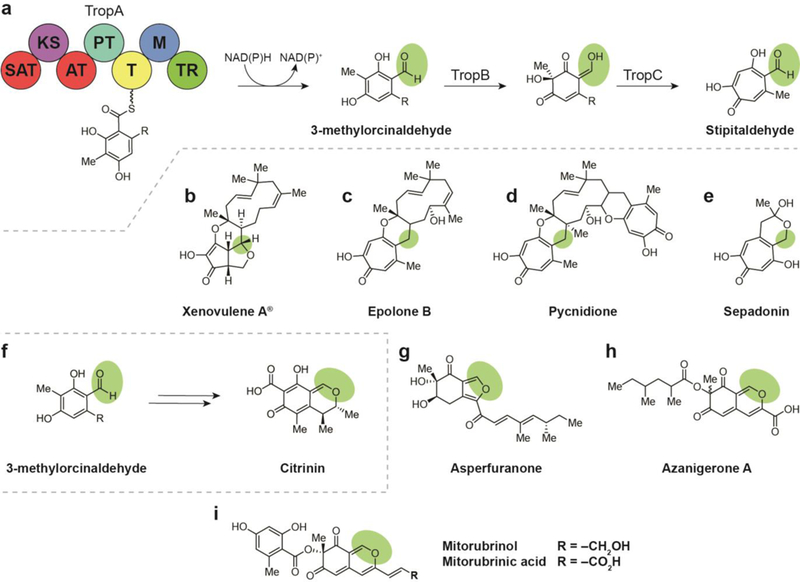
(a) Biosyntheses of natural products arising from a reductively released orcinaldehyde intermediate include the tropolones, which are biosynthesized via a tropolone intermediate (a-e), and the azaphilones, which arise from the cyclization of reductively released 3- methylorcinaldehyde to the bicyclic isochromene (f-h). (a) Terminal thioester reductase release of 3-methylorcinaldehyde leads to formation of stipitaldehyde. (b) Structure of xenovulene A. (c) Structure of epolone B. (d) Structure of pycnidione. (e) Structure of sepadonin. (f) Terminal thioester reductase release of 3-methylorcinaldehyde leads to formation of citrinin. (g) Structure of asperfuranone. (h) Structure of azanigerone A. (i) Structures of mitorubrinol and mitorubrinic acid.
