Fig. 7.
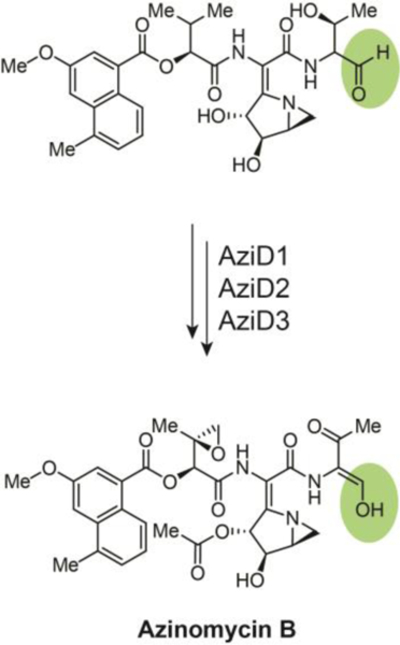
A two-electron reductive release of the aldehyde pre-azinomycin B precedes tautomerization to an enol that is stabilized through conjugation with the adjacent carbonyl, the product of threonine alcohol oxidation by AziD2, a putative acyl-CoA dehydrogenase.
