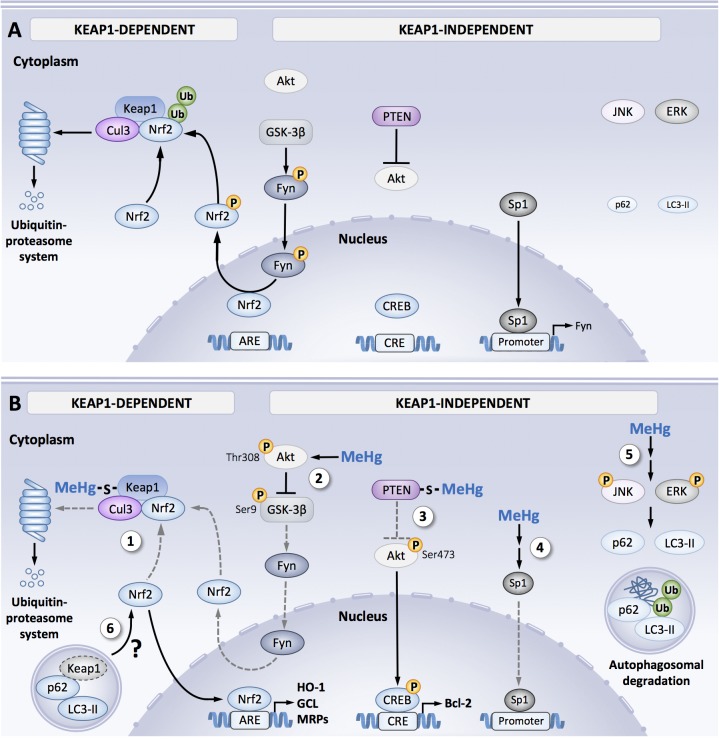
FIGURE 1.
Signal orchestration against MeHg toxicity. (A) Under non-stressed conditions, Nrf2 is captured by Keap1 and ubiquitinated by Cul3 in the cytosol that leads to degradation through ubiquitin–proteasome system, resulting in the inhibition of Nrf2 translocation from the cytoplasm to nucleus. Fyn is phosphorylated by GSK-3β, leading to Fyn nuclear localization. Fyn phosphorylates nuclear Nrf2, which leads to nuclear export and degradation of Nrf2. Sp1 is a transcription factor of Fyn. Steady state level of p62 and LC3-II expression under basal activity of JNK and ERK. (B) Cellular protective responses to MeHg. MeHg covalently modifies Keap1 through Cys151 and/or Cys319, leading to inhibition of Nrf2 degradation. As a result, Nrf2 translocates into nucleus and the interacts with a partner protein sMaf, resulting in formation of heterodimer that binds to the antioxidant response element (ARE), thereby upregulating its downstream genes (e.g., HO-1, GCL, and MRP) (1). MeHg induces phosphorylation of GSK-3β at Ser9 mediated by activated Akt at Thr308. Since this inactive form of GSK-3β is unable to phosphorylate Fyn, substantial retain of nuclear Nrf2 coupled to diminished nuclear translocation of Fyn occurs (2). MeHg covalently modifies PTEN, resulting in inhibition of its catalytic activity, thereby phosphorylating Akt through Thr473 and tits downstream transcription factor CREB, which binds to the cAMP-response element (CRE), leading to up-regulation of anti-apoptotic protein Bcl-2 (3). MeHg reduces Sp1 protein level and thus down-regulates Sp1-dependent target genes such as fyn (4). Phosphorylation of JNK and ERK mediated by MeHg increases p62 and LC3-II expressions, thereby promotes autophagosomal degradation of misfolded/damaged proteins (5). Possible linkage between MeHg-induced MAPK activation and Nrf2 upregulation via p62/LC3-II-mediated autophagosomal degradation of Keap1 (6). Dotted gray lines indicate processes disrupted by MeHg exposure.