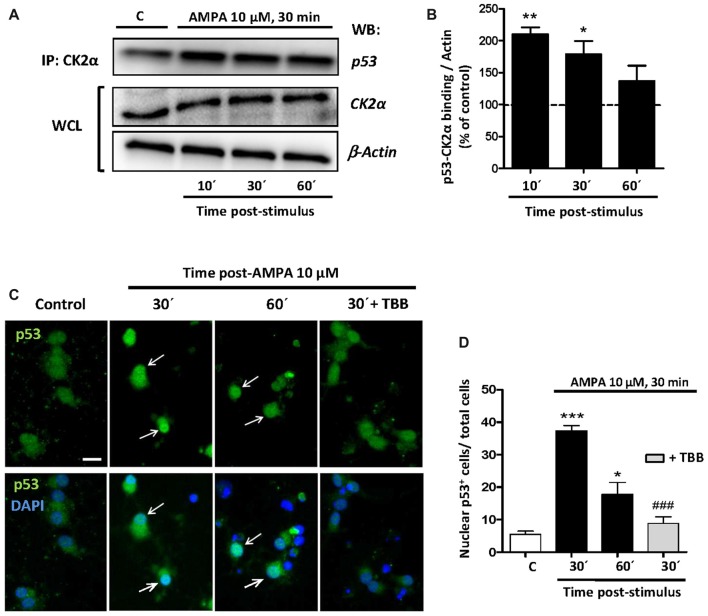
Figure 7.
CK2 directly interacts with p53 under conditions of AMPA-induced excitotoxicity in oligodendrocytes. (A) Oligodendrocytes in culture were subjected to excitotoxic insult and cell lysates were obtained at 10, 30 and 60 min after AMPA receptor activation. Co-immunoprecipitation assays were performed in the presence of rabbit polyclonal anti-CK2α antibody (IP: CK2α) and the immunoprecipitates were analyzed by WB using a mouse anti-p53 antibody. CK2α and β-actin were measured as internal loading controls in WCL. The image corresponds to one representative co-immunoprecipitation assay where it is observed that activation of AMPA receptors in oligodendrocytes provokes a direct interaction between CK2 and p53. (B) Quantification of p53-CK2α binding after co-immunoprecipitation assay in the experimental conditions indicated (n = 3; *p < 0.05; **p < 0.01 respect to control). (C) Immunofluorescence analysis of total p53 expression after AMPA receptors activation in absence or presence of CK2 inhibitor TBB. After AMPA treatment, oligodendrocytes were fixed, processed for immunofluorescence with an anti- total p53 antibody (p53 FL-393; green) and nuclei were stained with DAPI (blue). Images show that the increase in p53 activation and its translocation to the nuclei, examples indicated by arrows, was reduced by the CK2 inhibitor TBB. Scale bar, 10 μm. (D) Quantification of the number of p53 nuclear events determined by counting the cells with p53 in DAPI-positive region as fraction of total cells present in each condition. Cell counts were performed on a minimum of 10 independent fields (30 fields/3 coverslips/treatment) of images captured with a 20× objective, and all experimental conditions were always paired with corresponding internal controls (*p < 0.05; ***p < 0.001 respect to control; ###p < 0.001 respect to AMPA after 30 min).