Table 3.
Summary of % FPDmax changes at 30 min post compound addition for all compounds, all cell types and across all participating sites.
| Test article concentrations (μM) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Compound | Site/Cell type | Trend | ETPC-Free (uM) | Dose 1 | Dose 2 |
type |
Dose 3 |
type |
Dose 4 |
type |
| HIGH RISK | ||||||||||
| Disopyramide | Site 1, CDI |  |
0.7 | 0.1 | 1 | – | 10 | † | 100 | †† |
| Disopyramide | Site 2, CDI | 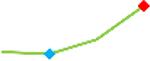 |
0.7 | 0.1 | 1 | – | 10 | † | 100 | †† |
| Disopyramide | Site 3, CDI | 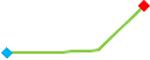 |
0.7 | 0.1 | 1 | – | 10 | † | 100 | †† |
| Disopyramide | Site 1, AXG |  |
0.7 | 0.1 | 1 | – | 10 | † | 100 | †† |
| Disopyramide | Site 2, AXG | 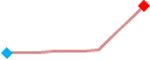 |
0.7 | 0.1 | 1 | – | 10 | – | 100 | † |
| Disopyramide | Site 4, AXG | 0.7 | 0.1 | 1 | – | 10 | – | not tested | ||
| Disopyramide | Site 2, CLS | 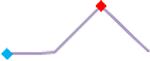 |
0.7 | 0.1 | 1 | – | 10 | – | 100 | †† |
| Disopyramide | Site 4, CLS |  |
0.7 | 0.1 | 1 | – | 10 | † | not tested | |
| Disopyramide | Site 2, PLR |  |
0.7 | 0.1 | 1 | – | 10 | † | 100 | †† |
| Quinidine | Site 1, CDI |  |
3 | 0.95 | 3 | †† | 9.49 | †† | 30 | quiescence |
| Quinidine | Site 2, CDI |  |
3 | 0.95 | 3 | † | 9.49 | † | 30 | † |
| Quinidine | Site 3, CDI |  |
3 | 0.95 | 3 | †† | 9.49 | †† | 30 | quiescence |
| Quinidine | Site 1, AXG |  |
3 | 0.95 | 3 | † | 9.49 | †† | 30 | quiescence |
| Quinidine | Site 2, AXG |  |
3 | 0.95 | 3 | – | 9.49 | † | 30 | † |
| Quinidine | Site 4, AXG |  |
3 | 0.95 | 3 | – | 9.49 | † | 30 | †† |
| Quinidine | Site 2, CLS | 3 | 0.95 | 3 | – | 9.49 | quiescence | 30 | quiescence | |
| Quinidine | Site 4, CLS |  |
3 | 0.95 | 3 | †† | 9.49 | quiescence | 30 | quiescence |
| Quinidine | Site 2, PLR | 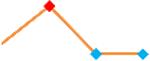 |
3 | 0.95 | 3 | † | 9.49 | † | 30 | † |
| D,I Sotalol | Site 1, CDI |  |
15 | 0.1 | 1 | – | 10 | †† | 100 | †† |
| D,l Sotalol | Site 2, CDI |  |
15 | 0.1 | 1 | – | 10 | †† | 100 | †† |
| D,l Sotalol | Site 3, CDI |  |
15 | 0.1 | 1 | – | 10 | †† | 100 | †† |
| D,l Sotalol | Site 1, AXG |  |
15 | 0.1 | 1 | – | 10 | – | 100 | †† |
| D,l Sotalol | Site 2, AXG |  |
15 | 0.1 | 1 | – | 10 | – | 100 | †† |
| D,l Sotalol | Site 4, AXG | 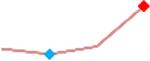 |
15 | 0.1 | 1 | – | 10 | – | 100 | †† |
| D,l Sotalol | Site 2, CLS | 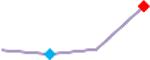 |
15 | 0.1 | 1 | – | 10 | – | 100 | †† |
| D,l Sotalol | Site 4, CLS | 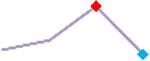 |
15 | 0.1 | 1 | – | 10 | – | 100 | †† |
| D,l Sotalol | Site 2, PLR |  |
15 | 0.1 | 1 | – | 10 | †† | 100 | †† |
| Vandetanib | Site 1, CDI | 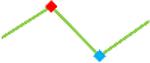 |
0.3 | 0.01 | 0.1 | – | 1.00 | † | 10 | †† |
| Vandetanib | Site 2, CDI | 0.3 | 0.01 | 0.1 | 1.00 | – | 10 | quiescence | ||
| Vandetanib | Site 3, CDI | 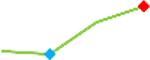 |
0.3 | 0.01 | 0.1 | – | 1.00 | † | 10 | †† |
| Vandetanib | Site 1, AXG | 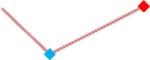 |
0.3 | 0.01 | 0.1 | – | 1.00 | – | 10 | †† |
| Vandetanib | Site 2, AXG |  |
0.3 | 0.01 | 0.1 | – | 1.00 | – | 10 | †† |
| Vandetanib | Site 4, AXG |  |
0.3 | 0.01 | 0.1 | – | 1.00 | – | 10 | †† |
| Vandetanib | Site 2, CLS |  |
0.3 | 0.01 | 0.1 | – | 1.00 | – | 10 | †† |
| Vandetanib | Site 4, CLS | 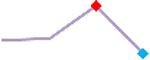 |
0.3 | 0.01 | 0.1 | – | 1.00 | – | 10 | †† |
| Vandetanib | Site 2, PLR |  |
0.3 | 0.01 | 0.1 | – | 1.00 | † | 10 | † |
| INTERMEDIATE RISK: | ||||||||||
| Astemizole | Site 1, CDI |  |
0.0003 | 0.0001 | 0.001 | – | 0.01 | †† | 0.1 | †† |
| Astemizole | Site 3, CDI | 0.0003 | 0.0001 | 0.001 | – | 0.01 | † | 0.1 | †† | |
| Astemizole | Site 1, AXG |  |
0.0003 | 0.0001 | 0.001 | – | 0.01 | †† | 0.1 | †† |
| Chlorpromazine | Site 1, CDI | 0.03 | 0.10 | 0.30 | – | 0.95 | – | 3.00 | – | |
| Chlorpromazine | Site 1, AXG | 0.03 | 0.10 | 0.30 | – | 0.95 | – | 3.00 | – | |
| Cisapride | Site 1, CDI |  |
0.003 | 0.003 | 0.01 | – | 0.03 | † | 0.1 | †† |
| Cisapride | Site 1, AXG | 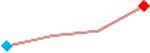 |
0.003 | 0.003 | 0.01 | – | 0.03 | †† | 0.1 | †† |
| Clarithromycin | Site 1, CDI | 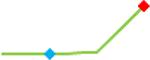 |
1.21 | 0.1 | 1 | – | 10 | †† | 100 | †† |
| Clarithromycin | Site 1, AXG |  |
1.21 | 0.1 | 1 | – | 10 | †† | 100 | †† |
| Domperidone | Site 2, CDI | 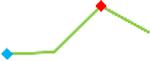 |
0.02 | 0.003 | 0.03 | – | 0.3 | †† | 3 | †† |
| Domperidone | Site 2, AXG |  |
0.02 | 0.003 | 0.03 | – | 0.3 | †† | 3 | †† |
| Domperidone | Site 2, CLS |  |
0.02 | 0.003 | 0.03 | – | 0.3 | †† | 3 | †† |
| Domperidone | Site 2, PLR |  |
0.02 | 0.003 | 0.03 | – | 0.3 | †† | 3 | †† |
| Droperidol | Site 2, CDI |  |
0.02 | 0.03 | 0.1 | – | 0.32 | † | 1 | † |
| Droperidol | Site 2, AXG |  |
0.02 | 0.03 | 0.1 | – | 0.32 | – | 1 | †† |
| Droperidol | Site 2, CLS | 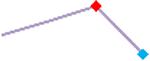 |
0.02 | 0.03 | 0.1 | – | 0.32 | † | 1 | †† |
| Droperidol | Site 2, PLR |  |
0.02 | 0.03 | 0.1 | – | 0.32 | †† | 1 | †† |
| Ondansetron | Site 3, CDI |  |
0.37 | 0.03 | 0.3 | – | 3.00 | † | 30.00 | † |
| Ondansetron | Site 4, AXG | 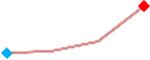 |
0.37 | 0.03 | 0.3 | – | 3.00 | – | 30.00 | †† |
| Ondansetron | Site 4, CLS |  |
0.37 | 0.03 | 0.3 | – | 3.00 | †† | 30.00 | quiescence |
| Pimozide | Site 2, CDI | 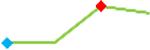 |
0.0004 | 0.001 | 0.003 | – | 0.01 | † | 0.03 | † |
| Pimozide | Site 3, CDI | 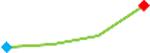 |
0.0004 | 0.001 | 0.003 | – | 0.01 | – | 0.03 | † |
| Pimozide | Site 2, AXG | 0.0004 | 0.001 | 0.003 | – | 0.01 | – | 0.03 | †† | |
| Pimozide | Site 4, AXG | 0.0004 | 0.001 | 0.003 | – | 0.01 | – | 0.03 | – | |
| Pimozide | Site 2, CLS | 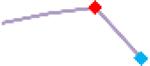 |
0.0004 | 0.001 | 0.003 | – | 0.01 | – | 0.03 | †† |
| Pimozide | Site 4, CLS |  |
0.0004 | 0.001 | 0.003 | – | 0.01 | †† | 0.03 | †† |
| Pimozide | Site 2, PLR | 0.0004 | 0.001 | 0.003 | – | 0.01 | †† | 0.03 | †† | |
| Risperidone | Site 3, CDI | 0.002 | 0.003 | 0.01 | – | 0.03 | – | 0.10 | – | |
| Risperidone | Site 4, AXG | 0.002 | 0.003 | 0.01 | – | 0.03 | no sg ch | 0.10 | – | |
| Risperidone | Site 4, CLS | 0.002 | 0.003 | 0.01 | – | 0.03 | no sg ch | 0.10 | – | |
| Terfenadine | Site 2, CDI | 0.0003 | 0.001 | 0.01 | – | 0.1 | – | 1 | quiescence | |
| Terfenadine | Site 2, AXG | 0.0003 | 0.001 | 0.01 | – | 0.1 | – | 1 | quiescence | |
| Terfenadine | Site 4, AXG | 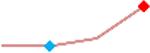 |
0.0003 | 0.001 | 0.01 | – | 0.1 | – | 1 | †† |
| Terfenadine | Site 2, CLS | 0.0003 | 0.001 | 0.01 | – | 0.1 | – | 1 | quiescence | |
| Terfenadine | Site 4, CLS | 0.0003 | 0.001 | 0.01 | – | 0.1 | – | 1 | †† | |
| Terfenadine | Site 2, PLR | 0.0003 | 0.001 | 0.01 | – | 0.1 | quiescence | 1 | quiescence | |
| LOW RISK | ||||||||||
| Diltiazem | Site 1, CDI | 0.13 | 0.01 | 0.1 | – | 1.00 | – | 10.00 | – | |
| Diltiazem | Site 1, AXG | 0.13 | 0.01 | 0.1 | – | 1.00 | quiescence | 10.00 | quiescence | |
| Diltiazem | Site 4, AXG | 0.13 | 0.01 | 0.1 | – | 1.00 | – | 10.00 | quiescence | |
| Diltiazem | Site 4, CLS | 0.13 | 0.01 | 0.1 | – | 1.00 | quiescence | 10.00 | quiescence | |
| Loratadine | Site 1, CDI | 0.0005 | 0.001 | 0.003 | – | 0.01 | – | 0.03 | – | |
| Loratadine | Site 1, AXG | 0.0005 | 0.001 | 0.003 | – | 0.01 | – | 0.03 | – | |
| Loratadine | Site 4, AXG | 0.0005 | 0.001 | 0.003 | – | 0.01 | – | 0.03 | – | |
| Loratadine | Site 4, CLS | 0.0005 | 0.001 | 0.003 | – | 0.01 | – | 0.03 | – | |
| Metoprolol | Site 4, AXG | 1.80 | 3.17 | 10.01 | – | 31.65 | no sg ch | 100 | – | |
| Metoprolol | Site 4, CLS | 1.80 | 3.17 | 10.01 | – | 31.65 | – | 100 | †† | |
| Mexiletine | Site 3, CDI | 2.50 | 0.1 | 1 | – | 10 | no sg ch | 100 | – | |
| Mexiletine | Site 4, AXG | 2.50 | 0.1 | 1 | – | 10 | – | 100 | – | |
| Mexiletine | Site 4, CLS | 2.50 | 0.1 | 1 | – | 10 | – | 100 | †† | |
| Nifedipine | Site 2, CDI | 0.01 | 0.001 | 0.01 | – | 0.1 | – | 1 | – | |
| Nifedipine | Site 2, AXG | 0.01 | 0.001 | 0.01 | – | 0.1 | – | 1 | – | |
| Nifedipine | Site 2, CLS | 0.01 | 0.001 | 0.01 | – | 0.1 | quiescence | 1 | quiescence | |
| Nifedipine | Site 2, PLR | 0.01 | 0.001 | 0.01 | – | 0.1 | – | 1 | – | |
| Nitrendipine | Site 2, CDI | 0.003 | 0.01 | 0.03 | – | 0.09 | – | 0.3 | – | |
| Nitrendipine | Site 2, AXG | 0.003 | 0.01 | 0.03 | – | 0.09 | – | 0.3 | – | |
| Nitrendipine | Site 2, CLS | 0.003 | 0.01 | 0.03 | quiescence | 0.09 | quiescence | 0.3 | quiescence | |
| Nitrendipine | Site 2, PLR | 0.003 | 0.01 | 0.03 | – | 0.09 | – | 0.3 | – | |
| Ranolazine | Site 2, CDI | 1.95 | 0.1 | 1 | – | 10 | – | 100 | quiescence | |
| Ranolazine | Site 3, CDI | 1.95 | 0.1 | 1 | – | 10 | – | 100 | †† | |
| Ranolazine | Site 2, AXG | 1.95 | 0.1 | 1 | – | 10 | – | 100 | quiescence | |
| Ranolazine | Site 2, CLS | 1.95 | 0.1 | 1 | – | 10 | – | 100 | †† | |
| Ranolazine | Site 2, PLR | 1.95 | 0.1 | 1 | – | 10 | – | 100 | †† | |
| Tamoxifen | Site 1, CDI | 0.02 | 0.1 | 0.3 | – | 0.95 | – | 3 | – | |
| Tamoxifen | Site 2, CDI | 0.02 | 0.1 | 0.3 | – | 0.95 | – | 3 | – | |
| Tamoxifen | Site 3, CDI | 0.02 | 0.1 | 0.3 | – | 0.95 | – | 3 | – | |
| Tamoxifen | Site 1, AXG | 0.02 | 0.1 | 0.3 | – | 0.95 | – | 3 | – | |
| Tamoxifen | Site 2, AXG | 0.02 | 0.1 | 0.3 | – | 0.95 | – | 3 | – | |
| Tamoxifen | Site 2, CLS | 0.02 | 0.1 | 0.3 | – | 0.95 | – | 3 | – | |
| Tamoxifen | Site 2, PLR | 0.02 | 0.1 | 0.3 | – | 0.95 | – | 3 | – | |
| Verapamil | Site 1, CDI | 0.05 | 0.001 | 0.01 | – | 0.1 | – | 1 | quiescence | |
| Verapamil | Site 3, CDI | 0.05 | 0.001 | 0.01 | – | 0.1 | – | 1 | – | |
| Verapamil | Site 1, AXG | 0.05 | 0.001 | 0.01 | – | 0.1 | – | 1 | quiescence | |
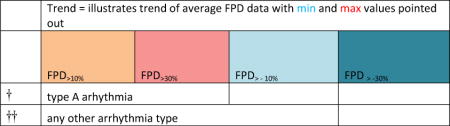
“Trend” lines represent average data per compound, n= 4 or 5 replicates each, with min (blue dot) and max (red dot) values.
represents type A arrhythmia as specified in the HESI Myocyte Phase II Validation Study Protocol. All other types of/combinations of arrhythmia observed are marked with
(type B, C, D arrhythmias listed Myocyte Phase II Validation Study Protocol). Colour shades represent the first dose where FPDmax was > or <10% change (or >< 30%, in case compounds were more potent).
