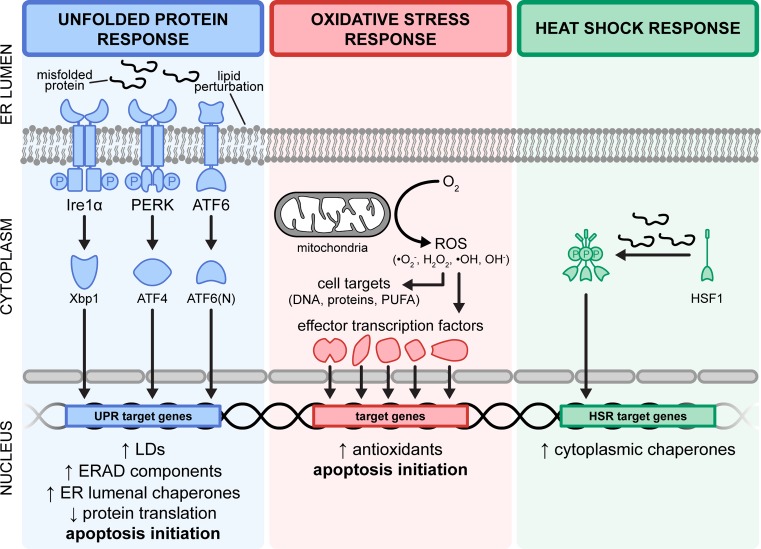
Figure 2. Fundamental activation mechanism of major cellular stress responses.
(Left panel) The UPR is activated by ER stress conditions (i.e. ER membrane perturbation and aberrant protein folding within the ER lumen). These stressors are affected by three distinct axes, namely Ire1α, PERK and ATF6. The cognate transcription factors, Xbp1, ATF4 and ATF6(N), then translocate into the nucleus to modulate gene expression including those of ER luminal chaperones and ERAD components. UPR activation concurrently attenuates global protein translation and induces LD formation to restore homeostasis. (Middle panel) Oxidative stressors in the form of reactive oxygen species are generated through energy metabolism. Accumulation of ROS potentially induces a cascade of events that damages DNA, proteins and long-chain PUFAs. This cellular threat is mitigated by the up-regulation of antioxidants that neutralise ROS. The failure to restore homeostasis under conditions of ER and oxidative stress ultimately results in apoptosis. (Right panel) In contrast with the UPR, the presence of misfolded proteins in the cytoplasm as well as elevated temperatures activates the heat shock response (HSR). Under these conditions, the monomeric HSF1 sensor forms the homotrimeric HSF1 transcription factor that translocates into the nucleus to increase expression of cytoplasmic protein chaperones to subsequently aid in the refolding and processing of misfolded client substrates. Abbreviations: ERAD, ER-associated degradation; PUFA: polysaturated fatty acid; ROS, reactive oxygen species.