| 1 |
Hydroxypropyl cellulose (HPC) |
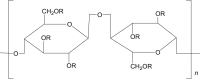 |
-
•
Not specified by WHO for human use
-
•
Lethal dose, 50% (LD50) (rat intravenous administration: 0.25 g/kg)
|
* |
52, 68
|
| 2 |
Hypromellose (HPMC) |
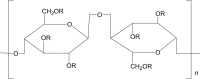 |
|
* |
52, 68
|
| 3 |
Leucine |
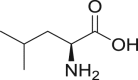 |
-
•
It is generally regarded as a nontoxic and non-irritant material
-
•
Only moderately toxic by the subcutaneous route.
-
•
LD50 (rat, IP): 5.379 g/kg
|
IV-52.6% |
52, 69, 70
|
| 4 |
Poloxamer F68, F127 |
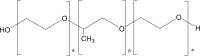 |
-
•
No adverse effects at IV administration up to 0.5 g/kg/day in dogs
-
•
0.5 g/kg/day to rabbits
-
•
LD50 (mouse, IP): 1 g/kg,
-
•
LD50 (rat, IV): 7.5 g/kg
|
Poloxamer F68: |
52, 68
|
| IM-0.2%, |
| IV-0.6%, |
| 5 |
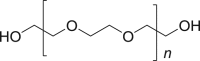
Polyethyleneglycol (PEG) 400 |
 |
-
•
The WHO has set an estimated acceptable daily intake of polyethylene glycols at up to 10 mg/kg body-weight
-
•
LD50 (mouse, IV): 8.6 g/kg
-
•
LD50 (rat, IV): 7.3 g/kg
|
IM-20%, |
52, 68
|
| IV-75.58% |
| 6 |
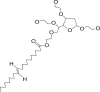
Polysorbate 80 |
 |
-
•
The WHO has set an estimated acceptable daily intake for polysorbates 25 mg/kg body-weight
-
•
LD50 (mouse, IV): 4.5 g/kg
-
•
LD50 (rat, IV): 1.8 g/kg
|
IM-12%, |
52, 68
|
| IV infusion-58.5% |
| 7 |
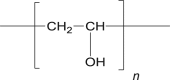
Polyvinylalcohol (PVA) |
 |
-
•
PVA is generally considered a nontoxic material
-
•
LD50 (mouse, oral): 14.7 g/kg
-
•
LD50 (rat, oral): > 20 g/kg
|
Intravitreal-0.12 mg |
52, 68
|
| 8 |
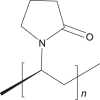
Povidon |
 |
-
•
A temporary acceptable daily intake for povidone has been set by the WHO at up to 25 mg/kg body-weight.
-
•
LD50 (mouse, IP): 12 g/kg
|
IV-0.2%, IM-0.9% |
52, 68
|
| 9 |
2-Pyrrolidone |
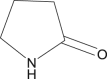 |
-
•
Poisonous by IV route. Toxic by ingestion, skin contact, and IP routes. It is an experimental teratogenic, mutagenic additive
-
•
LD50 (mouse, IV): 0.155 g/kg
-
•
LD50 (mouse, oral): 5.13 g/kg
-
•
LD50 (rabbit, SC): 8.0 g/kg
-
•
LD50 (rat, IP): 2.472 g/kg
-
•
LD50 (rat, IV): 0.0805 g/kg
|
SC-25.85% |
52, 68
|
| 10 |
Sodium lauryl sulfate (SLS) |
 |
|
* |
52, 68
|
| 11 |
Cross povidone |
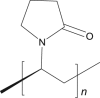 |
|
IM-0.02% |
52, 68
|
| 12 |
Chitosan |
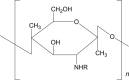 |
|
* |
52 |
| 13 |
Cyclodextrin |
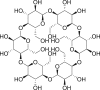 |
-
•
Cyclodextrin-α: LD50 (rat, IP): 1.0 g/kg
-
•
LD50 (rat, IV): 0.79 g/kg
-
•
Cyclodextrin-β: LD50 (mouse, IP): 0.33 g/kg
-
•
LD50 (mouse, SC): 0.41 g/kg
-
•
LD50 (rat, IP): 0.36 g/kg
-
•
LD50 (rat, IV): 1.0 g/kg
-
•
LD50 (rat, oral): 18.8 g/kg
-
•
LD50 (rat, SC): 3.7 g/kg
-
•
Cyclodextrin-γ: LD50 (rat, IP): 4.6 g/kg
-
•
LD50 (rat, IV): 4.0 g/kg
-
•
LD50 (rat, oral): 8.0 g/kg
|
IV-5% |
52, 68
|
| 14 |
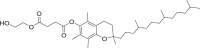
d-α-Tocopheryl polyethylene glycol 1000 succinate (vitamin E TPGS) |
 |
|
IV-225 mg/L |
52, 68
|
| 15 |
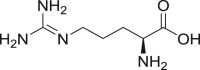
Arginine |
 |
|
IM-78%, |
52, 68, 71, 72
|
| IV-39.5% |
| 16 |
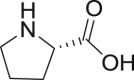
Proline |
 |
|
IV infusion-35.6% |
73, 51, 68
|
| 17 |
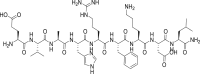
Serum albumin |
 |
-
•
Essentially nontoxic and non-irritant
-
•
LD50 (monkey, IV): >12.5 g/kg
-
•
LD50 (rat, IV): >12.5 g/kg
|
IV-2% |
52, 68
|
| 18 |
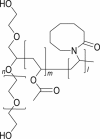
Soluplus®
|
 |
* |
* |
74, 75
|
















