FIG 7.
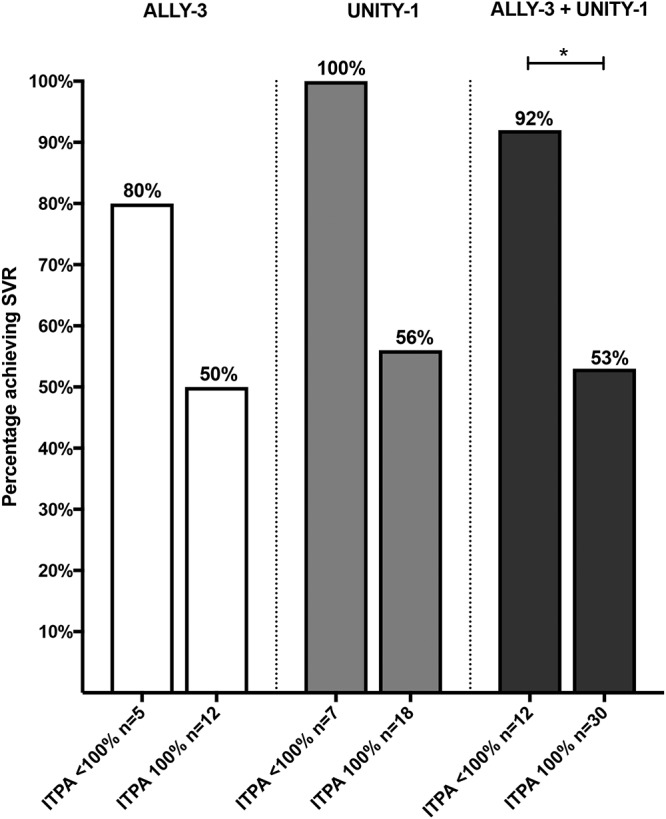
Percentage of patients with normal (100%) or reduced (<100%) ITPase activity having baseline, pretreatment NS5A resistance-associated substitutions (RASs; also known as resistance-associated variants, or RAVs) that achieved SVR following treatment with daclatasvir and sofosbuvir without ribavirin for 12 weeks in the ALLY-3 study (HCV genotype 3-infected patients with noncirrhosis or cirrhosis) and with daclatasvir, asunaprevir, and beclabuvir without ribavirin for 12 weeks in the UNITY-1 trial (HCV genotype 1-infected noncirrhotic patients). Statistical significance was determined using Fisher's exact test (*, P < 0.05).
