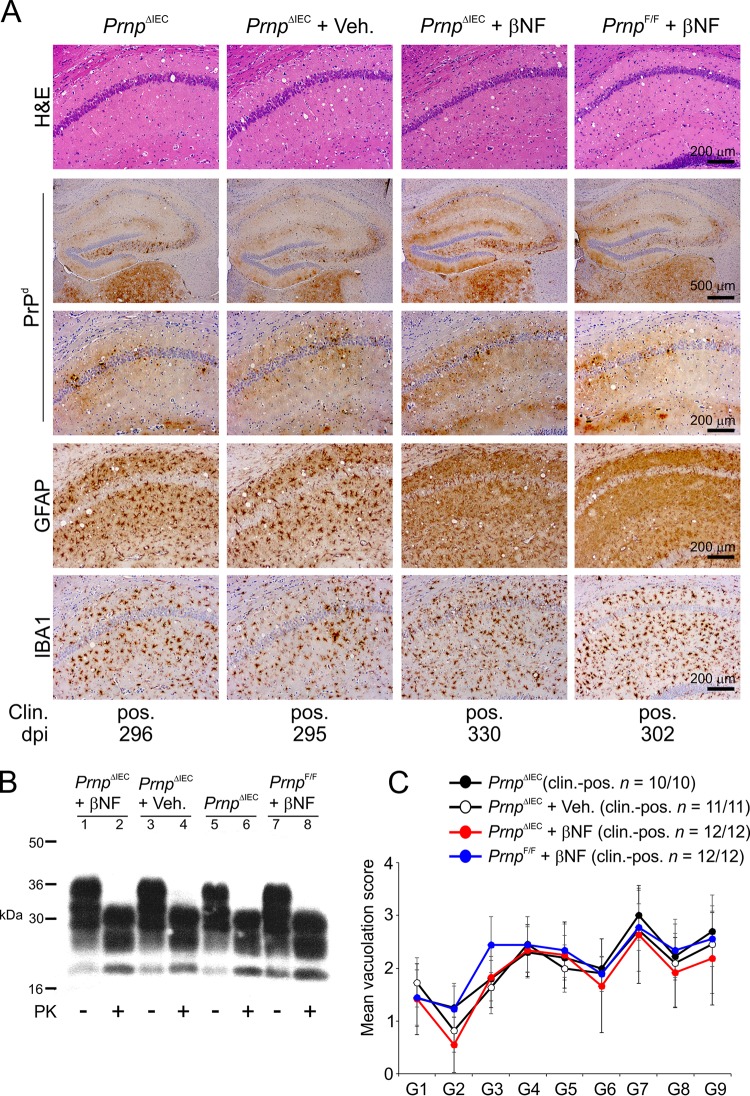
FIG 4.
Intestinal epithelial cell-restricted Prnp ablation does not influence development of the histopathological signs of prion disease in the brains of clinically affected mice. Female PrnpΔIEC mice were treated with βNF for 5 days to specifically ablate Prnp expression in intestinal epithelial cells. Untreated PrnpΔIEC mice and PrnpΔIEC mice treated with the vehicle alone (Veh.) were used as controls. Fourteen days later, the mice were orally exposed to ME7 scrapie prions and culled when they succumbed to clinical prion disease. (A) High levels of spongiform pathology (hematoxylin and eosin [H&E] stain), heavy accumulations of disease-specific PrP (PrPd) (brown), reactive astrocytes expressing GFAP (brown), and active microglia expressing Iba-1 (brown) were detected in the brains of all orally exposed mice with clinical prion disease. Clin., clinical prion disease status; pos., clinically positive. Individual survival times are shown (dpi, days postinfection). Sections were counterstained with hematoxylin to detect cell nuclei (blue). (B) Immunoblot analysis of brain tissue homogenates confirms the presence of high levels of prion-specific, relatively proteinase K (PK)-resistant PrPSc within the brains of the clinically affected mice from each group. Samples were treated in the presence (+) or absence (−) of PK before electrophoresis. After PK treatment, a typical three-band pattern was observed between molecular mass values of 20 and 30 kDa, representing unglycosylated, monoglycosylated, and diglycosylated isomers of PrP (in order of increasing molecular mass). (C) The severity and distribution of the spongiform pathology (vacuolation) within each brain were scored on a scale of 1 to 5 in nine gray matter areas: dorsal medulla (G1), cerebellar cortex (G2), superior colliculus (G3), hypothalamus (G4), thalamus (G5), hippocampus (G6), septum (G7), retrosplenial and adjacent motor cortex (G8), and cingulate and adjacent motor cortex (G9). Each point represents the mean vacuolation score ± SD (n = 10 to 12 mice/group).