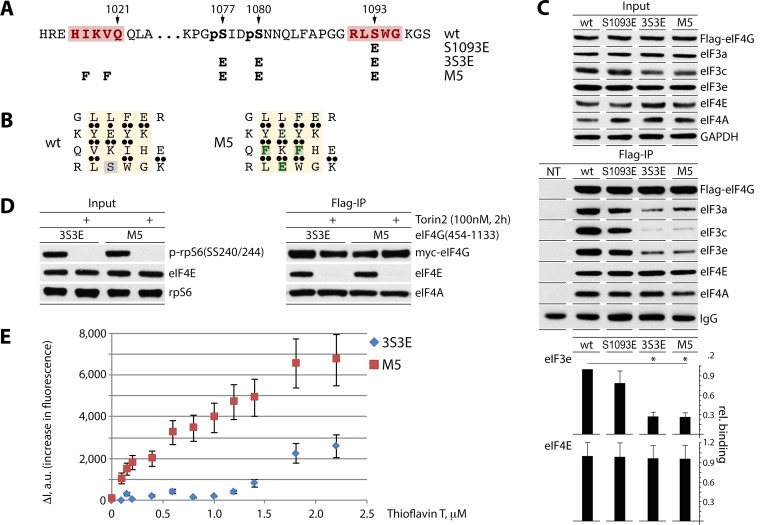
FIG 4.
Design of the 4-stranded β-sheet with a high-affinity ThT binding site and fluorescent titration of eIF4G:eIF4A complexes by ThT. (A and B) Mutations S1093E, 3S3E and M5 in eIF4G(454-1133) (A). M5 carries a high-affinity ThT site in the putative 4-stranded β-sheet (see the text). Substituted amino acids are colored green (B). (C) HEK293 cells were transfected for expression of the indicated fragments (16 h) and synchronized by Th block (24 h). Cell lysates were subjected to immunoblotting (top panel) or anti-Flag IP/immunoblotting (middle panel) with the indicated antibodies. Results from Flag co-IP of eIF3e/eIF4E were quantified and averaged between 3 tests (bottom panel); error bars represent SEM, and asterisks represent Student t test results (P < 0.05). (D and E) Fluorescent titration of eIF4G:eIF4A complexes by ThT. HEK293 cells were transfected for expression of 3S3E and M5 fragments (16 h) and synchronized by Th block (24 h), and Torin2 was added 2 h prior to lysis to block eIF4E:eIF4G binding (D). For isolation of eIF4G:eIF4A complexes, cell lysates were subjected to sequential anti-Flag IP/Flag-peptide elution and anti-Myc IP/Myc-peptide elution. ThT:eIF4G(454-1133) binding isotherms were obtained by measuring the increase in dye fluorescence intensity (ΔI) (480 nm) relative to that in buffer (E). Fluorescence titrations were performed in triplicate; error bars represent SEM.