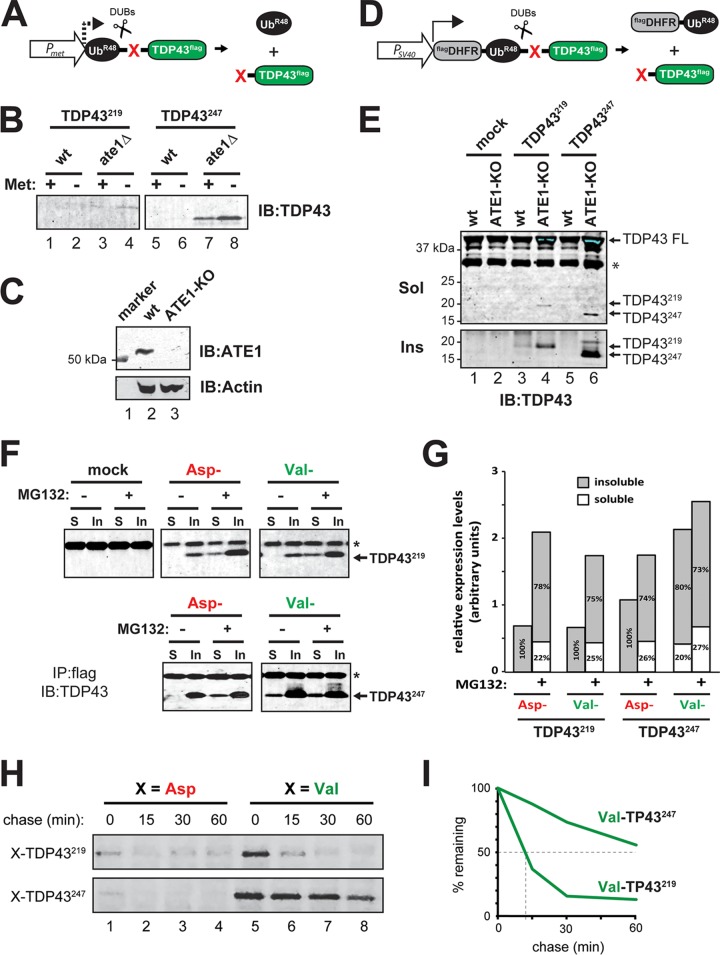
FIG 2.
Differential degradation of TDP43219 and TDP43247 in the absence of ATE1 and the Arg/N-end rule pathway. (A) TDP43219 and TDP43247 were expressed in yeast by using the ubiquitin fusion technique (71). Cotranslational cleavage by cellular deubiquitylases (DUBs) yields UbR48 (which contains a K48R mutation to prevent its participation in polyubiquitin chains that direct proteins to the proteasome for degradation) and a test fragment (TDP43219 or TDP43247) bearing a specified N-terminal amino acid (red X) and a C-terminal FLAG epitope tag. The PMet promoter was used to induce expression when grown in methionine-lacking medium. (B) SDS-PAGE and immunoblotting (IB) using an anti-TDP43 antibody to detect steady-state levels of TDP43219 and TDP43247 expressed in wild-type (wt) and ATE1-lacking (ate1Δ) yeast. Expression was induced by growth in medium lacking methionine (Met). (C) ATE1 is detected by immunoblotting at ∼55 kDa in lysates of wild-type N2a cells but not in N2a cells that have undergone CRISPR/Cas9-mediated ATE1 ablation. The bottom panel shows actin used as a loading control. (D) The ubiquitin reference technique (URT), derived from the Ub fusion technique (71). Cotranslational cleavage by DUBs of a URT-based fusion produces, at an initially equimolar ratio, a stable internal reference protein such as FLAGDHFR-UbR48, a FLAG-tagged derivative of the mouse dihydrofolate reductase, and a C-terminally FLAG-tagged test fragment (TDP43219 or TDP43247) bearing a specified N-terminal amino acid (red X). Moderate expression in mammalian cells was achieved by using the PSV40 promoter. (E) Steady-state levels of TDP43219 and TDP43247 expressed in wild-type N2a cells and those that had undergone CRISPR-mediated knockout of ATE1 (ATE1-KO). Full-length (FL), endogenous mouse TDP43 as well as exogenously added TDP43219 and TDP43247 were detected in detergent-soluble (Sol) and urea-soluble (Ins) fractions by SDS-PAGE and immunoblotting using an anti-TDP43 antibody. (F) Partitioning of detergent-soluble and -insoluble TDP43219 and TDP43247 bearing N-terminal Asp or N-terminal Val (not arginylated by ATE1), which were expressed in HEK293T cells. Cells were treated with 5 μM MG132 for 24 h, as indicated, prior to lysis. Fragments were immunoprecipitated (IP) from the soluble (S) and insoluble (In) fractions using an anti-FLAG antibody and detected by immunoblotting using an anti-TDP43 antibody. Asterisks represent the antibody light chain. (G) Graphical representation of the densitometric analysis of bands in panel F to show relative expression levels and the percentages of the total found in the soluble or insoluble fraction. (H) Pulse-chase analysis of TDP43219 and TDP43247 bearing either N-terminal Asp or Val in HEK293T cells. Fragments were labeled with [35S]Met-Cys, followed by a chase, preparation of extracts, immunoprecipitation with anti-FLAG, SDS-PAGE, and autoradiography. (I) Quantification of N-terminal Val-bearing fragments in panel H.