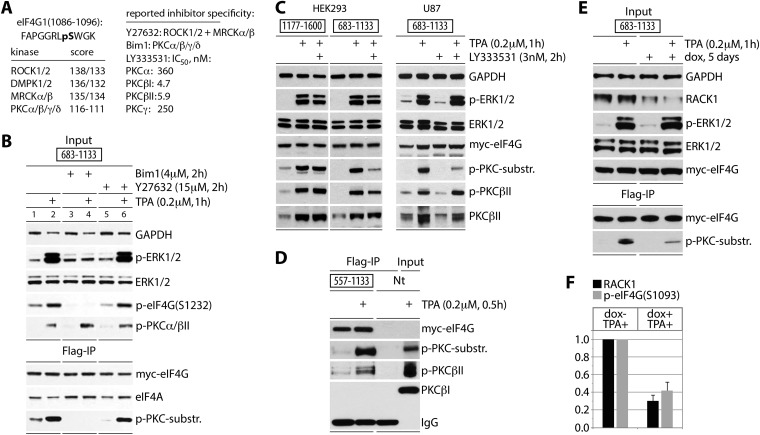
FIG 2.
eIF4G(S1093) is phosphorylated by RACK1:PKCβII. (A) Sequence context of eIF4G(S1093), quantitative computational prediction of kinases (http://www.phosphonet.ca), and specificity of inhibitors used in this study (20, 21). (B) Bim-1 inhibits TPA-induced eIF4G(S1093) phosphorylation. HEK293 cells were transfected for expression of the indicated tagged fragments (16 h), serum starved (24 h), pretreated with or without the indicated inhibitor (2 h), and treated with TPA. Cell lysates were subjected to immunoblotting (top panel) or anti-Flag IP/immunoblotting (bottom panel) with the indicated antibodies. The experiment was repeated three times with consistent results; results of a representative test are shown. (C) The PKCβ-specific inhibitor LY33353 (20) blocks TPA-induced eIF4G(S1093) phosphorylation. HEK293 and U87 cells were treated as described for panel B. Cell lysates were analyzed by immunoblotting with the indicated antibodies. Three test runs yielded similar results; results of a representative assay are shown. (D) TPA-dependent co-IP of active PKCβII with eIF4G. Nontransfected cells (Nt) were used as a negative IP control. (E) Dox-inducible RACK1 depletion prevents eIF4G(S1093) phosphorylation. HeLa cells were Dox induced (5 days), transfected with eIF4G(683-1133) as for panel B, and TPA stimulated. Cell lysates were analyzed by immunoblotting (input) and Flag IP followed by immunoblotting as shown. (F) Quantification of RACK1 depletion and reduction of p-eIF4G(S1093) was normalized by setting the value of +TPA/−Dox to 1; error bars represent SEM. For panels D and E, three repeat assays yielded similar outcomes, and results of representative tests are depicted.