Halophytes are important coastal plants often used for the remediation of saline coastal soils. Limonium sinense is well known for its medical properties and phytoremediation of saline soils. However, excessive exploitation and utilization have made the wild resource endangered. The use of endophytic and rhizosphere bacteria may be one of the suitable ways to solve the problem. This study was undertaken to develop approaches to improve the growth of L. sinense using endophytes. The application of actinobacterial endophytes ameliorated salt stress damage of the host via complex physiological and molecular mechanisms. The results also highlight the potential of using habitat-adapted, symbiotic, indigenous endophytic bacteria to enhance the growth and ameliorate abiotic stress damage of host plants growing in special habitats.
KEYWORDS: halophyte, endophytes, rhizobacteria, Illumina MiSeq, diversity, Glutamicibacter, transcriptome
ABSTRACT
Plant-associated microorganisms are considered a key determinant of plant health and growth. However, little information is available regarding the composition and ecological function of the roots' and leaves' bacterial microbiota of halophytes. Here, using both culture-dependent and culture-independent techniques, we characterized the bacterial communities of the roots and leaves as well as the rhizosphere and bulk soils of the coastal halophyte Limonium sinense in Jiangsu Province, China. We identified 49 representative bacterial strains belonging to 17 genera across all samples, with Glutamicibacter as the most dominant genus. All Glutamicibacter isolates showed multiple potential plant growth promotion traits and tolerated a high concentration of NaCl and a wide pH range. Interestingly, further inoculation experiments showed that the Glutamicibacter halophytocola strain KLBMP 5180 isolated from root tissue significantly promoted host growth under NaCl stress. Indeed, KLBMP 5180 inoculation increased the concentrations of total chlorophyll, proline, antioxidative enzymes, flavonoids, K+, and Ca2+ in the leaves; the concentrations of malondialdehyde (MDA) and Na+ were reduced. A transcriptome analysis identified 1,359 and 328 differentially expressed genes (DEGs) in inoculated seedlings treated with 0 and 250 mM NaCl, respectively. We found that pathways related to phenylpropanoid and flavonoid biosynthesis and ion transport and metabolism might play more important roles in host salt stress tolerance induced by KLBMP 5180 inoculation compared to that in noninoculated leaves. Our results provide novel insights into the complex composition and function of the bacterial microbiota of the coastal halophyte L. sinense and suggest that halophytes might recruit specific bacteria to enhance their tolerance of harsh environments.
IMPORTANCE Halophytes are important coastal plants often used for the remediation of saline coastal soils. Limonium sinense is well known for its medical properties and phytoremediation of saline soils. However, excessive exploitation and utilization have made the wild resource endangered. The use of endophytic and rhizosphere bacteria may be one of the suitable ways to solve the problem. This study was undertaken to develop approaches to improve the growth of L. sinense using endophytes. The application of actinobacterial endophytes ameliorated salt stress damage of the host via complex physiological and molecular mechanisms. The results also highlight the potential of using habitat-adapted, symbiotic, indigenous endophytic bacteria to enhance the growth and ameliorate abiotic stress damage of host plants growing in special habitats.
INTRODUCTION
There is now clear evidence that plants live in complex associations with microorganisms and that the microbiota has a profound influence on plant nutrition, development, and environmental adaptation (1–3). In turn, the microbiota community structure is also influenced by the host plant species, the organ containing the microbes, the location of the plant, and other environmental factors (4). Specifically, plant microbiota may stimulate growth, elicit a systemic defense against pathogens and pests, and enhance the resistance to abiotic stressors, including drought, salinity, and heavy metals (5–8). Microbes in the rhizo- and endospheres may mediate plant growth and stress tolerance directly via mechanisms such as nitrogen fixation, soil phosphate solubilization, and the production of phytohormones or indirectly by producing siderophores, antibiotics, 1-aminocyclopropane-1-carboxylic acid deaminase (ACCD), and hydrolytic enzymes (9, 10).
The plant rhizosphere is a complex ecosystem inhabited by numerous microbes whose abundance and activities are mediated by plant root exudates (11). The interactions between the plant and the microbiota are complex. To clarify the interaction mechanisms and ecological functions of plant-associated microbiota, it is necessary to investigate their diversity and composition (12). To date, studies of plant microbiota have primarily focused on crop plants and other model plants (13–15); information about bacterial microbiota structure and function in plants inhabiting special and extreme habitats remains limited.
It is estimated that over one-third of the arable land worldwide is salt affected (16). Soil salinity is a serious abiotic stressor that influences plant growth and reduces crop productivity. Coastal salt marshes are typical salt-affected ecosystems, with high salt concentrations and low organic matter content (17). Halophytic plants cope with salt stress through complex physiological responses and molecular regulation mechanisms (18). Limonium sinense, a salt-secreting halophyte dicot endemic to China, is distributed mainly in coastal areas of southern China; this species has recently been listed as endangered. This halophyte has been used as a “pioneering plant” in the phytoremediation of saline soils (19). L. sinense is also a commercially important traditional Chinese medicine. It has been used to treat hemorrhages and may have other beneficial medical properties, such as antitumor, antiviral, and immunomodulatory properties (20, 21). Several studies have suggested that the habitat-adapted symbiotic microorganisms associated with plants growing in harsh environments might help these host plants tolerate abiotic stresses (22–24). Recently, halophyte-associated endophytes and rhizosphere microorganisms have been shown to increase the salt tolerance of the host plants (25–28). However, few details of halophyte microbiota composition are available, and the mechanisms by which halophyte-associated microbes increase salinity tolerance in halophytes remain unclear. As the plant microbiota is mainly influenced by plant species and the growth environment, we hypothesize that specimens of the halophyte L. sinense growing in coastal salt marshes likely possess habitat-adapted, symbiotic, indigenous microbial communities capable of increasing the salinity tolerance of the host plant.
Therefore, the aims of the present study are (i) to analyze the rhizo- and endospheric bacterial composition and structure of L. sinense using 16S rRNA gene-based high-throughput sequencing on the Illumina MiSeq platform, (ii) to isolate and identify rhizospheric and endophytic bacteria in L. sinense and to evaluate their potential as plant growth promoters (PGP) and for salt stress reduction, and (iii) to elucidate the physiological and molecular mechanisms modulated by PGPs via physiological and transcriptomic analyses of the L. sinense leaf.
RESULTS
Bacterial isolation and diversity.
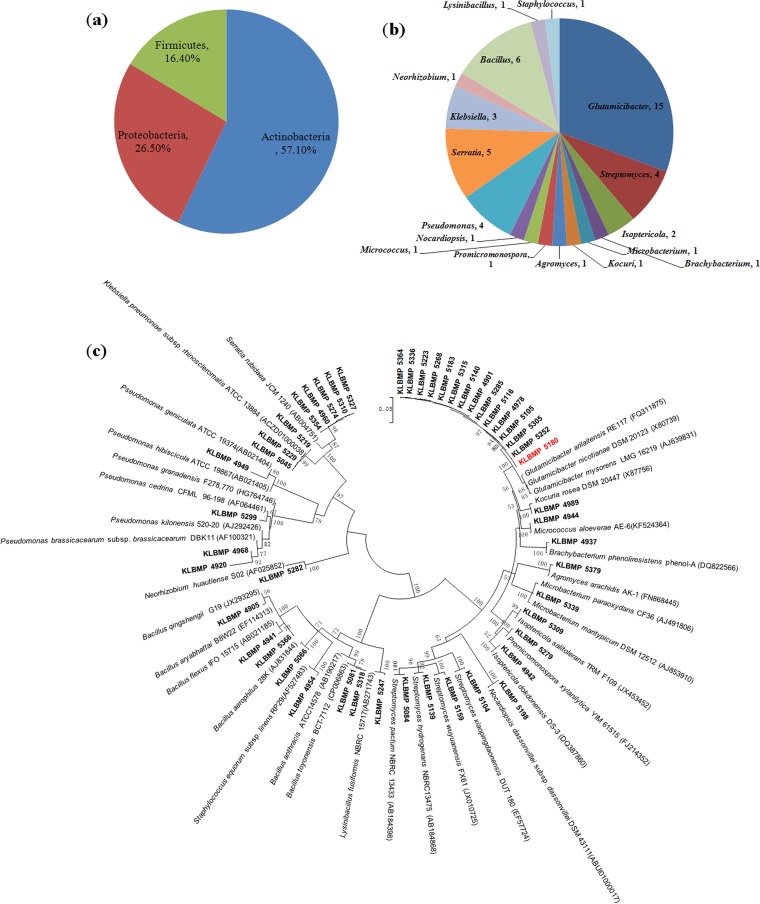
We cultivated 243 bacterial strains from the inner tissues of the roots and leaves of L. sinense and 105 strains from the rhizosphere soil samples. After the phenotypic classification of the isolates on the basis of morphological and cultural characters, we selected 49 representative strains (20 from the rhizosphere soil, 14 from the roots, and 15 from the leaves) for taxonomic analysis based on 16S rRNA gene sequencing (see Table S1 in the supplemental material). These 49 strains were distributed across three phyla (28 Actinobacteria, 13 Proteobacteria, and 8 Firmicutes) (Fig. 1a) and 17 genera. We found 10 genera in the phylum Actinobacteria, with Glutamicibacter (15 isolates), Streptomyces (4 isolates), and Isoptericola (2 isolates) having more than one representative isolate. In the phylum Firmicutes, we identified 3 genera, including Bacillus (6 isolates), Lysinibacillus (1 isolate), and Staphylococcus (1 isolate). Finally, we obtained 4 genera in the phylum Proteobacteria: Pseudomonas (4 isolates), Serratia (5 isolates), Klebsiella (3 isolates), and Neorhizobium (1 isolate) (Fig. 1b). Notably, the 16S rRNA gene sequences of the 15 Glutamicibacter isolates were highly similar (99.1% to 100% sequence identity) (Fig. 1c) and were most closely related to Glutamicibacter arilaitensis and Glutamicibacter nicotianae (Table S1), despite being isolated from different sources: rhizosphere soil (8 isolates), roots (5 isolates), and leaves (2 isolates) (Table 1).
FIG 1.
Taxonomic diversity of the obtained representative bacterial strains. (a) Composition percentages of the isolates at the phylum level. (b) Numbers of isolates at the genus level. (c) Neighbor-joining 16S rRNA gene tree of the isolates and their nearest phylogenetic type species. Bar, 0.05 substitution per nucleotide position.
TABLE 1.
Presence of plant growth promotion traits in Glutamicibacter isolates
| Origin | Strain | Growth on nitrogen-free medium | Phosphate solubilization | IAA production | ACCD production | pH range for growth | Tolerance of 1,700 mM NaCl |
|---|---|---|---|---|---|---|---|
| Rhizosphere soil | KLBMP 5364 | − | + | + | + | 5–10 | + |
| KLBMP 5336 | + | − | − | + | 4–10 | + | |
| KLBMP 5223 | + | − | − | + | 4–10 | + | |
| KLBMP 5268 | − | − | + | + | 5–10 | + | |
| KLBMP 5315 | − | − | − | + | 5–10 | + | |
| KLBMP 5285 | + | − | − | + | 4–10 | + | |
| KLBMP 5305 | + | − | − | + | 4–10 | + | |
| KLBMP 5252 | + | − | − | + | 4–10 | + | |
| Root | KLBMP 4901 | + | + | − | + | 5–10 | + |
| KLBMP 5183 | + | − | + | + | 4–10 | + | |
| KLBMP 4978 | − | − | + | + | 4–10 | + | |
| KLBMP 5116 | + | − | + | + | 4–10 | + | |
| KLBMP 5180 | + | − | + | + | 4–10 | + | |
| Leaf | KLBMP 5140 | + | − | − | + | 4–10 | + |
| KLBMP 5105 | + | − | − | + | 4–10 | + |
Bacterial community composition by culture-independent method.
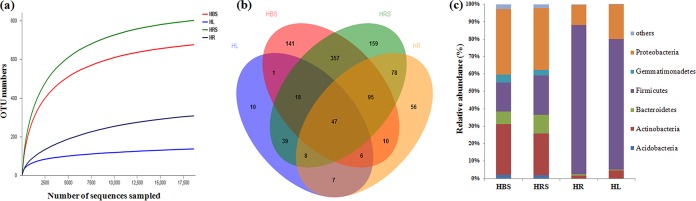
We obtained 122,687 high-quality sequences, 18,744 to 42,422 for each sample (see Table S3). The coverage values, diversity indices, and rarefaction curves that we calculated (Fig. 2a) suggested that rhizosphere and bulk soil bacterial communities were more diverse than endophytic communities. We identified 1,032 operational taxonomic units (OTUs) using the 97% sequence similarity cutoff, ranging from 136 to 801 per sample (Table S3); 47 OTUs were found in all samples (Fig. 2b). We identified 23 different phyla across all four sources (bulk soil, rhizosphere soil, roots, and leaves). The predominant phyla (>1%) were Acidobacteria, Actinobacteria, Bacteroidetes, Firmicutes, Gemmatimonadetes, and Proteobacteria (Fig. 2c), but their relative abundances varied by source. Firmicutes were most predominant in both the roots (85.8%) and the leaves (74.9%), but much less common in the bulk soil (16.7%) and the rhizosphere soil (22.5%). Proteobacteria and Actinobacteria were the most abundant phyla in the bulk soil and rhizosphere soil samples. Our results therefore indicated that the roots and leaves have similar bacterial communities, as do the two types of soil samples.
FIG 2.
Comparison of operational taxonomic units (OTUs) in bacterial communities from different samples: bulk soil (HBS), Limonium sinense roots (HR) and leaves (HL), and rhizosphere soil (HRS). (a) Rarefaction curves depicting the effect of 3% dissimilarity on the number of OTUs identified. (b) Venn diagram showing the sources of OTUs. (c) Bacterial community composition at the phylum level.
We recovered 233 different known genera across all four sources. Enterococcus and Bacillus were the most dominant genera in roots (56.65% and 11.99%, respectively) and in leaves (49.57% and 10.22%, respectively). Longispora and Enterococcus were the most common genera in the bulk soil (10.97% and 9.28%, respectively). The most dominant known genus in the rhizosphere soil was Bacillus (16.71%), followed by Paenarthrobacter (3.75%) and Enterococcus (2.46%) (see Fig. S1). As actinobacterial isolates dominated the strains we cultivated, we also analyzed the actinobacterial communities of the root, leaf, rhizosphere soil, and bulk soil samples. We detected 41 known actinobacterial genera, including many rare genera such as Kibdelosporangium, Ilumatobacter, Longispora, Marmoricola, and Quadrisphaera. Paenarthrobacter was the most frequently detected known actinobacterial genus in rhizosphere soils (15.8% within the Actinobacteria phylum). Streptomyces was most common in the roots (49.3%), Marmoricola (43.9%) in the leaves, and Longispora (37.6%) in the bulk soil samples (see Table S4). Interestingly, one OTU (classified as Glutamicibacter nicotianae) found in the rhizosphere soil, roots, and leaves had 100% sequence match with the 16S rRNA genes of the 15 Glutamicibacter isolates (Table 2).
TABLE 2.
OTUs clustered at 100% similarity within the genera Arthrobacter, Glutamicibacter, and Paenarthrobactera
| OTU | Classification | No. of OTU sequences |
No. of isolatesb | |||
|---|---|---|---|---|---|---|
| Bulk soil | Rhizosphere soil | Root | Leaf | |||
| 1 | Arthrobacter tumbae | 9 | 21 | 0 | 0 | 0 |
| 2 | Paenarthrobacter, unclassified | 48 | 698 | 1 | 7 | 0 |
| 3 | Glutamicibacter nicotianae | 0 | 66 | 1 | 1 | 5 (HR), 2 (HL), 8 (HRS) |
Clustering was performed with aligned sequences from 16S rRNA gene of obtained strains and sequenced samples. The genera Glutamicibacter and Paenarthrobacter originally belonged to the genus Arthrobacter and were reclassified from species of the genus Arthrobacter in 2016 by Busse (45).
HR, roots; HL, leaves; HRS, rhizosphere soil.
Plant growth promotion traits and effect of Glutamicibacter strains under salt stress.
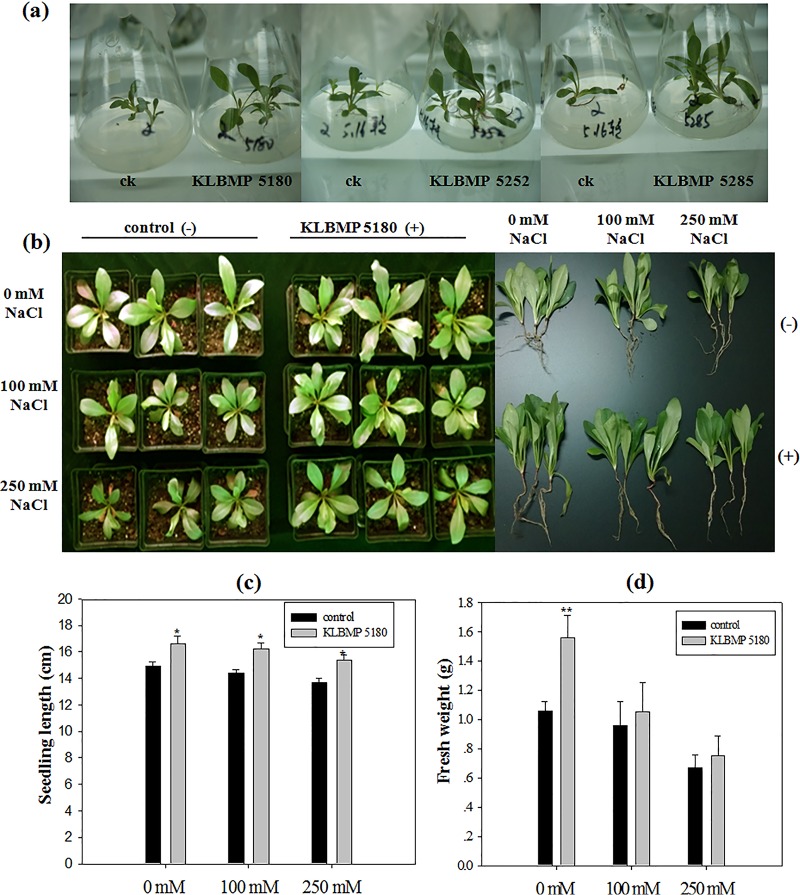
As most of the isolates we sequenced were members of the genus Glutamicibacter, we selected the 15 strains in this genus for the investigation of PGP characters. Eleven strains grew on nitrogen-free agar medium, two strains solubilized phosphate, and six strains produced indole-3-acetic acid (IAA) (Table 1). All 15 strains produced ACCD, tolerated a high concentration of NaCl, and showed growth for a wide range of pHs (Table 1). As all 15 Glutamicibacter isolates showed higher 16S rRNA gene sequence identity and similar PGP traits, three isolates (KLBMP 5180, KLBMP 5252, and KLBMP 5285) were randomly selected to evaluate the potential PGP ability by using an L. sinense seed inoculation experiment. After 2 months of growth on Murashige and Skoog (MS) medium with 85 mM NaCl, the seedlings inoculated with any of the three selected Glutamicibacter strains grew bigger than the noninoculated seedlings (Fig. 3a). We further evaluated the PGP ability of strain KLBMP 5180 with a pot experiment, as it has been identified as a novel species, proposed as Glutamicibacter halophytocola by the polyphasic taxonomic approach (29). The seedlings inoculated with strain KLBMP 5180 grew significantly better both with and without NaCl stress (Fig. 3b). The total seedling length and fresh weight were increased compared with those of noninoculated plants (Fig. 3c and d).
FIG 3.
Effects of bacterial inoculation on the growth of Limonium sinense seedlings stressed with NaCl. (a) Plants grown on MS agar with 0.5% NaCl and inoculated with strains KLBMP 5180, KLBMP5252, and KLBMP 5285. ck, noninoculated control. (b) Aerial view and whole seedlings of control (noninoculated) plants and those inoculated with strain KLBMP 5180 exposed to different levels of NaCl stress. (c) Seedling lengths. (d) Total fresh weight of seedlings. Graphed values are means (n = 3 per treatment); error bars indicate standard errors (SEs). *, P < 0.05; **, P < 0.01 between noninoculated control plants and inoculated plants.
Physiological responses of L. sinense.
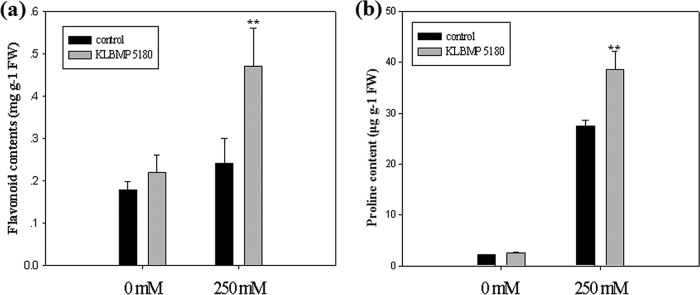
We found no significant differences in the leaf chlorophyll or proline contents between inoculated and noninoculated plants grown without NaCl stress (Table 3; Fig. 4b). However, when stressed with 250 mM NaCl, chlorophyll and proline in the leaves increased 1.15- and 1.4-fold, respectively, in plants inoculated with KLBMP 5180 compared to those in noninoculated plants (Table 3; Fig. 4b). We measured the malondialdehyde (MDA) content of the leaves to determine whether the inoculation with KLBMP 5180 reduced lipid peroxidation and the degree of cell damage. We found that the inoculation with KLBMP 5180 significantly decreased MDA accumulation: 37.7% less when treated with 0 NaCl and 28.6% less when stressed with 250 mM NaCl (P < 0.01 for both) (Table 3). The flavonoid content in the leaves stressed with 250 mM NaCl was also increased significantly after the inoculation with KLBMP 5180 compared to that in noninoculated plants (P < 0.01) (Fig. 4a). The activity of three stress-related enzymes (catalase [CAT], peroxidase [POD], and phenylalanine ammonia-lyase [PAL]) increased under salt stress. The inoculation with KLBMP 5180 increased CAT and POD significantly in both the presence and absence of NaCl (P < 0.01): after inoculation, POD activity increased 133.9% without NaCl and 76.9% with 250 mM NaCl (Table 3). PAL activity also increased significantly when the plant was exposed to 250 mM NaCl (P < 0.01).
TABLE 3.
Effects of inoculation with strain KLBMP 5180 on the leaf total chlorophyll, oxidative stress responsive species, and activity of antioxidant enzymes in plants treated with 250 mM NaCl and untreated plantsa
| Treatment | Total chlorophyll (mg · g−1 FW) | MDA (μmol · g−1 FW) | PAL (U · g−1 FW) | POD (U · g−1 FW) | CAT (U · mg−1 protein) |
|---|---|---|---|---|---|
| Control | 1.34 ± 0.10 | 7.44 ± 0.40 | 11.45 ± 2.65 | 798.33 ± 52.87 | 14.45 ± 1.3 |
| Control plus strain | 1.35 ± 0.06 | 4.64 ± 0.27** | 13.84 ± 2.01 | 1867.01 ± 112.09** | 21.34 ± 1.93** |
| 250 mM NaCl | 1.12 ± 0.08 | 11.21 ± 1.38 | 18.31 ± 2.60 | 1237.37 ± 143.11 | 18.60 ± 1.26 |
| 250 mM NaCl plus strain | 1.26 ± 0.03* | 8.01 ± 1.07** | 34.64 ± 4.68** | 2188.50 ± 139.83** | 24.12 ± 1.16** |
Significant differences between noninoculated control plants and inoculated plants: *, P < 0.05; **, P < 0.01.
FIG 4.
Effects of inoculation with strain KLBMP 5180 on properties of Limonium sinense leaves in plants treated with 250 mM NaCl and untreated plants. (a) Total flavonoids. (b) Proline. Graphed values are means (n = 3 per treatment); error bars indicate SEs. **, P < 0.01 between noninoculated control plants and inoculated plants.
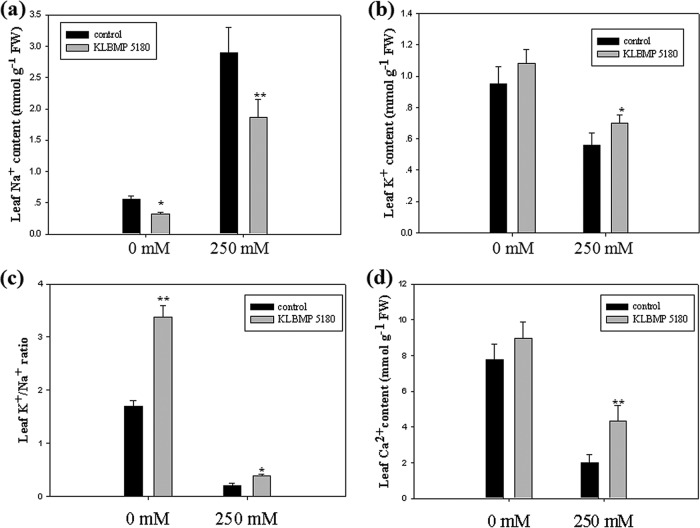
We compared ion accumulation in L. sinense leaves between plants inoculated with KLBMP 5180 and noninoculated plants. Na+ content decreased significantly after the inoculation with KLBMP 5180 in unstressed and NaCl-stressed plants compared to that in noninoculated plants (decreases of 42.3% and 35.4%, respectively) (Fig. 5a). K+ and Ca2+ in the leaves increased significantly in plants exposed to 250 mM NaCl compared to those in noninoculated plants (increases of 25% and 90%, respectively) (Fig. 5b and d). KLBMP 5180 significantly improved the K+/Na+ ratio in the leaves, irrespective of salt content (Fig. 5c).
FIG 5.
Effects of inoculation with strain KLBMP 5180 on ions in Limonium sinense leaves in plants treated with 250 mM NaCl and untreated plants. (a) Na+ content. (b) K+ content. (c) K+/Na+ ratio. (d) Ca2+ content. Graphed values are means (n = 3 per treatment); error bars indicate SEs. *, P < 0.05; **, P < 0.01 between noninoculated control plants and inoculated plants.
Transcriptomic profiles and identification of DEGs.
To identify potential molecular mechanisms underlying the enhanced salt tolerance of L. sinense induced by the inoculation with Glutamicibacter strain KLBMP 5180, we performed transcriptome sequencing (RNA-seq) analyses of L. sinense leaves inoculated with KLBMP 5180 and grown with or without salt stress. Each set of treatments was performed on three independent biological replicates. After removing adaptors and low-quality sequences, we obtained between 20.7 and 31.6 million clean reads from each of the 12 libraries; all Q30s were greater than 90%. We successfully mapped 84.1% to 87.9% of the clean reads from each library (see Table S5).
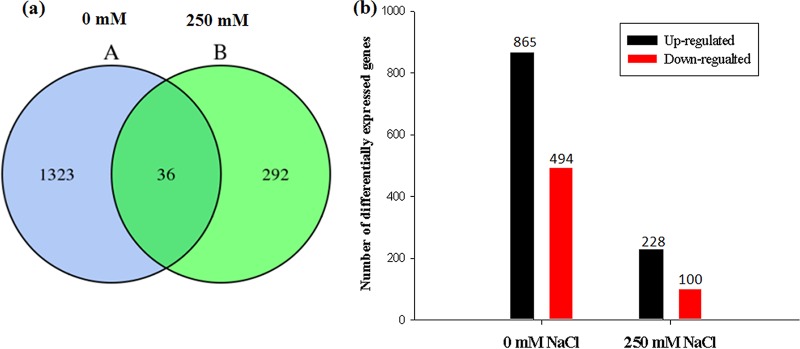
After the inoculation with KLBMP 5180, 1,359 differentially expressed genes (DEGs) were identified in the plants grown without NaCl stress, and 328 DEGs were identified in plants stressed with 250 mM NaCl. Of these, 36 DEGs were identified in both groups of plants: 1,323 DEGs only in plants grown without salt stress and 292 only in seedlings stressed with 250 mM NaCl (Fig. 6a). Of the DEGs identified in inoculated plants grown without salt stress, 865 were upregulated and 494 were downregulated compared to those in noninoculated plants; in inoculated plants treated with 250 mM NaCl, 228 DEGs were upregulated and 100 were downregulated compared to those in noninoculated plants (Fig. 6b).
FIG 6.
Differentially expressed genes (DEGs) in KLBMP 5180-inoculated and noninoculated plants treated with 0 and 250 mM NaCl. (a) Venn diagram indicating unique and shared DEGs (A, 0 mM NaCl; B, 250 mM NaCl). (b) DEGs up- and downregulated in inoculated plants treated with 0 and 250 mM NaCl compared to that of noninoculated plants.
Functional analysis of DEGs.
We used the Gene Ontology (GO) database to identify the function of the DEGs. The genes differentially expressed in the inoculated plants were assigned to three different GO categories: biological process, cellular location, and molecular function. We recovered 35 GO terms pertaining to the DEGs in the inoculated plants grown without salt and 28 GO terms in the inoculated seedlings stressed with 250 mM NaCl (see Fig. S2).
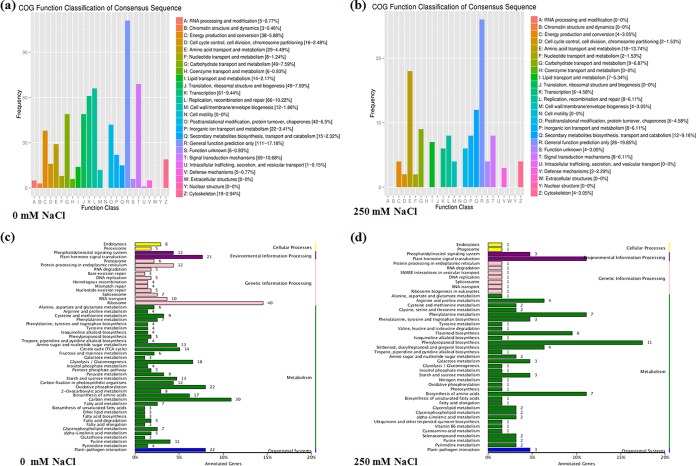
Identified DEGs were aligned to the Clusters of Orthologous Groups of proteins (COG) database for the classification and prediction of gene function. More than 17% of all genes differentially expressed in the inoculated plants could not be accurately annotated (“general function prediction only”). In the inoculated seedlings treated with 0 mM NaCl, the top three COG categories were “signal transduction mechanisms” (10.68%), “replication, recombination, and repair” (10.22%), and “transcription” (9.44%) (Fig. 7a). In the inoculated seedlings stressed with 250 mM NaCl, the top two categories were “amino acid transport and metabolism” (13.74%) and “secondary metabolites biosynthesis, transport, and catabolism” (9.16%) (Fig. 7b). This suggests that DEGs classified in these groups may be involved in the amelioration of NaCl stress damage in L. sinense by actinobacterium KLBMP 5180.
FIG 7.
COG (a and b) and KEGG (c and d) analyses of differentially expressed genes (DEGs) in Limonium sinense plants inoculated with strain KLBMP 5180 and treated with 0 mM NaCl (a and c) and 250 mM NaCl (b and d) relative to those in noninoculated plants.
We further analyzed KEGG pathways containing the identified DEGs. In inoculated plants grown without salt, 510 of the 1,359 DEGs mapped to the KEGG database, and two categories, “plant-pathogen interaction” and “phosphatidylinositol signaling system,” were significantly enriched (corrected P value, <0.05) (Fig. 7c). In inoculated plants treated with 250 mM NaCl, 103 of the 328 DEGs mapped to the KEGG database, and four different categories were significantly enriched (corrected P value, <0.05): phenylpropanoid biosynthesis, flavonoid biosynthesis, phenylalanine metabolism, and stilbenoid, diarylheptanoid, and gingerol biosynthesis (Fig. 7d; see also Table S6).
DEGs associated with the phenylpropanoid and flavonoid biosynthesis.
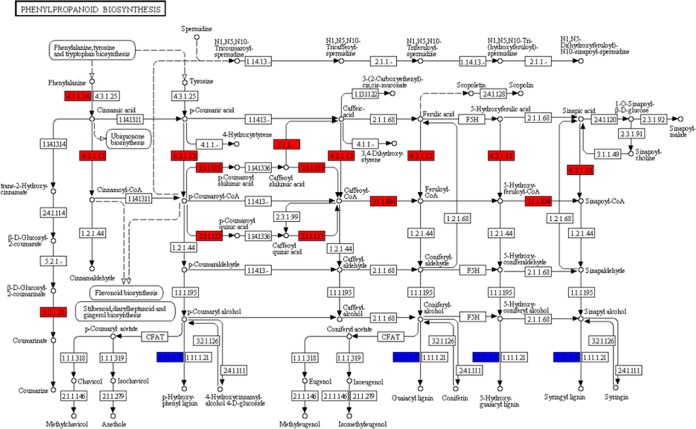
The KEGG pathway analysis indicated that 11 DEGs were involved in the phenylpropanoid pathway. DEGs encoding PAL, which initiates lignin biosynthesis from phenylalanine, were upregulated, while DEGs encoding POD, an enzyme involved in catalyzing the final synthesis step, exhibited mixed patterns of expression (Fig. 8). POD is an important antioxidant enzyme that protects plants by acting as a reactive oxygen species (ROS) scavenger. All six DEGs involved in flavonoid biosynthesis were upregulated (see Fig. S3). These results suggested that when L. sinense is exposed to salt stress, strain KLBMP 5180 might increase leaf lignin and flavonoid biosynthesis.
FIG 8.
Analysis of differentially expressed genes (DEGs) related to the phenylpropanoid biosynthesis pathway in Limonium sinense plants inoculated with strain KLBMP 5180 and treated with 250 mM NaCl compared to those in noninoculated plants treated with 250 mM NaCl. Red indicates upregulated DEGs; blue indicates DEGs with mixed patterns of regulation.
DEGs related to ion transport and metabolism and carbohydrate metabolism.
Several genes involved in ion transport and metabolism were differentially expressed in plants inoculated with strain KLBMP 5180. In the inoculated plants grown without salt, 14 transporter genes were upregulated and 11 were downregulated, including the genes encoding the cation transporter ATPase, the sodium/metabolite cotransporter, the organic anion transporter, and the sodium/potassium ion P-ATPase family transporter. In inoculated seedlings stressed with 250 mM NaCl, genes encoding the potassium transporter, the calcium-binding protein, the sulfate transporter, and the organic cation/carnitine transporter were upregulated. These results indicated that the ion transporter genes regulated by KLBMP 5180 help to maintain ion homeostasis in L. sinense leaves in response to salt stress.
We also analyzed the amino acid and trehalose metabolism pathways after KLBMP 5180 inoculation. We observed that the two genes encoding pyrroline-5-carboxylate synthase and delta-1-pyrroline-5-carboxylate synthase, which are responsible for the synthesis of proline biosynthetic precursors, were only upregulated in inoculated seedlings stressed with 250 mM NaCl. Furthermore, one gene involved in trehalose metabolism, encoding trehalose 6-phosphate phosphatase (TPP), was upregulated in inoculated plants stressed with 250 mM NaCl and downregulated in plants grown without salt (see Table S7).
DEGs related to TFs and plant hormone metabolism.
We identified 54 transcription factors (TFs) differentially expressed in inoculated plants grown without salt stress and 8 TFs differentially expressed in inoculated plants treated with 250 mM NaCl (Table S7). Among these differentially expressed TFs, high percentages of AP2, WRKY, bHLH, and MYB families were identified. Interestingly, the TF bHLH35 and the dehydration-responsive element-binding (DREB) protein 3 were only upregulated in inoculated plants stressed with 250 mM NaCl. Both of these TFs have been shown to regulate gene expression in plants in response to abiotic stressors, such as salt, drought, and low temperatures (30, 31). DEGs related to plant hormone signal transduction pathways ranked fifth and second of the top 50 KEGG pathways in inoculated plants (Fig. 7c and d). We identified 28 DEGs in the inoculated plants that were involved in plant hormone metabolism, including genes encoding auxin, gibberellin (GA), abscisic acid (ABA), ethylene (ET), brassinosteroid (BR), jasmonic acid (JA), and salicylic acid (SA). Several genes were upregulated only in the inoculated plants grown without salt: the genes encoding GID1 and TF (GA pathway), the genes encoding PYR/PYL and PP2C (the core components of ABA signaling), the gene encoding PR-1 (SA pathway), and the gene encoding TCH4 (BR pathway) (see Fig. S4a). The gene encoding JAZ (JA signaling pathway) was upregulated only in the inoculated seedlings stressed with 250 mM NaCl. The auxin-induced SAUR family protein gene was upregulated in all inoculated plants, while AUX/IAA exhibited mixed patterns of expression in inoculated plants stressed with 250 mM NaCl only (Fig. S4b). The gene encoding 1-aminocyclopropane-1-carboxylate (ACC) oxidase (ET biosynthesis pathway) was downregulated in all inoculated plants (Table S7). In addition, three genes involved in IAA biosynthesis were upregulated in inoculated plants: two genes encoding flavin-containing monooxygenase and indole-3-pyruvate monooxygenase in plants grown without salt, and one gene encoding flavin-containing monooxygenase in seedlings stressed with 250 mM NaCl.
Validation of differentially expressed genes by RT-qPCR.
To verify the reliability of DEGs identified with RNA-seq, we measured the gene expression of seven randomly selected DEGs with reverse transcription quantitative PCR (RT-qPCR). These seven DEGs included three genes upregulated and four downregulated in all inoculated plants. Our RT-qPCR results were consistent with the transcriptional profile data generated from RNA-seq (see Table S8).
DISCUSSION
Plant microbiomes are crucial to plant development, growth, and biotic or abiotic stress tolerance (32). To date, the microbiota patterns of the rhizo- and endospheres of various crops plants (e.g., maize and rice) and model plants (e.g., Arabidopsis thaliana and Populus) have been well characterized (7, 33–35). However, the details of the microbial communities associated with plants growing in saline environments, and the ecological functions of these communities, remain poorly understood.
Here, we characterized the bacterial microbiota associated with the rhizo- and endospheres of the typical coastal halophyte L. sinense, using both culture-dependent and culture-independent methods. The obtained bacterial strains fell into three phyla: Actinobacteria, Proteobacteria, and Firmicutes. Among the 17 different genera identified in the bacterial cultures, the most common were Glutamicibacter, Bacillus, and Streptomyces; many species in these genera are considered PGP agents in plants subjected to salt stress (36–38).
The analyses of OTUs and species diversity in the roots, leaves, rhizosphere, and bulk soil indicated that bulk soil and rhizosphere soil harbored more diverse bacterial communities than the roots or the leaves (see Table S3 in the supplemental material). Indeed, the bacterial communities of the roots and leaves were similar in composition, while the rhizosphere soil had the greatest bacterial diversity and species richness. This finding is in accordance with the reports of the bacterial microbiota of the coastal halophytes Limonium vulgare (39) and Messerschmidia sibirica (40). This is probably due to the exudates secreted by plant roots, which could be used as carbon and nitrogen sources for bacterial growth in the rhizosphere (41).
The bacterial phyla Proteobacteria, Actinobacteria, Firmicutes, and Bacteroidetes dominated across all sample sources, although the proportions varied among sources. This was approximately congruent with our culture-dependent results. Firmicutes was the most abundant phylum in the roots and leaves, whereas Proteobacteria and Actinobacteria were more dominant in the rhizosphere and bulk soil than in roots and leaves (Fig. 2c). Firmicutes, Proteobacteria, and Actinobacteria may exhibit profound roles in the adaptation of the host plant to different environments, as these phyla also dominated the microbiota of other halophytes (40, 42, 43). In the rhizosphere, soil, roots, and leaves, the dominant genera were Bacillus, Enterococcus, Paenarthrobacter, and Solibacillus, respectively. The differences in generic compositions across samples might indicate that plant tissues favor colonization by specific bacteria.
Endophytic bacterial communities in plants are thought to be recruited from the rhizosphere (44). We therefore focused on the genus Glutamicibacter, as this dominated the cultured strains and could also be isolated from rhizosphere soil, roots, and leaves (Table 1). Interestingly, the sequence of one OTU (putatively classified as G. nicotianae) had 100% sequence identity with 15 Glutamicibacter isolates and was found in the roots, the leaves, and the rhizosphere soil (Table 2). The two genera Glutamicibacter and Paenarthrobacter were reclassified recently from selected species of the genus Arthrobacter (45). Arthrobacter is a versatile genus of actinobacteria that inhabits a diverse range of environments. Many Arthrobacter species have been identified in the rhizospheres and endospheres of various plants; these species have been shown to act as PGPs, improving host plant nutrition, yield, and resistance to abiotic stressors (46–48). Our evaluation of the PGP traits of the 15 Glutamicibacter isolates identified here indicated that all 15 strains produced ACCD and tolerated a wide range of pH values and high concentrations of NaCl. To further clarify the potential ecological functions of Glutamicibacter strains, we performed inoculation experiments. Interestingly, we observed that all three strains of Glutamicibacter tested increased the growth of seedlings grown on MS medium and treated with 0.5% NaCl. One strain, KLBMP 5180, significantly increased the growth of the host plant when stressed with NaCl (P < 0.05) (Fig. 3). Our results, together with a recent analysis of root microbiota composition and function in the halophyte Suaeda salsa (49), support the previously published hypothesis that habitat-adapted symbiotic endophytes may make an important contribution to the host plant stress tolerance and fitness (22, 23). However, the PGP ability of strain KLBMP 5180 in natural salinity soil still needs to be validated in further studies.
The growth increase observed in inoculated host plants subjected to salt stress might be due to the multiple PGP traits of the inoculated strain. We therefore examined the physiological responses of host plants inoculated with KLBMP 5180 and subjected to salt stress. In comparison to those of salt-stressed noninoculated leaves, salt-stressed KLBMP 5180-inoculated leaves had more total chlorophyll, higher proline concentrations, and lower MDA concentrations; this indicated a decrease in salt-related oxidative damage to cell membranes. The activity of the antioxidative enzyme CAT increased in inoculated plants grown without salt; the activity of the antioxidative enzyme POD increased in inoculated seedlings stressed with 250 mM NaCl. This indicated that KLBMP 5180 reduces the osmotic and oxidative stress caused by NaCl. Similar effects were reported in maize (50–52) and white clover (53). The inoculation of L. sinense seedlings with KLBMP 5180 also significantly increased the efflux of Na+ and the uptake of K+ and Ca2+ compared to those in noninoculated plants, leading to an increase in the K+/Na+ ratio and an overall reduction in ionic stress in response to high salt concentrations. Our results are consistent with those of studies of wheat and the halophyte grass Puccinellia tenuiflora, where inoculation with the soil bacterium Bacillus subtilis GB03 increased K+ uptake and decreased Na+ accumulation under high-salt conditions (54, 55).
We used a transcriptome analysis to elucidate the molecular mechanisms involved in salinity resistance after inoculation with KLBMP 5180. We identified several DEGs, indicating that the encoded proteins played specific roles in promoting seedling growth and salt tolerance. We found that the “phenylpropanoid biosynthesis” and “flavonoid biosynthesis” pathways were significantly enriched in inoculated seedlings exposed to high concentrations of NaCl compared to those in noninoculated seedlings (Fig. 7c and d). Several DEGs involved in both pathways were significantly upregulated in inoculated seedlings treated with salt. In the phenylpropanoid pathway, we identified seven upregulated DEGs and four DEGs with mixed expression patterns in inoculated seedlings exposed to salt. Among these DEGs, we detected DEGs encoding PAL and POD, two key enzymes for lignin biosynthesis, which catalyze the initial and final synthesis steps, respectively (56). Lignin is a phenylpropanoid compound derived from phenylalanine. Previous studies have demonstrated that higher lignin deposition is induced in plants under alkaline salt conditions, which is an adaptation mechanism for plants to resist salinity-imposed stress (57, 58). Here, PAL and POD activity increased significantly after KLBMP 5180 inoculation (Table 3), which might indicate an increase in L. sinense leaf lignin biosynthesis via the phenylpropanoid pathway after treatment with NaCl.
Recent studies have demonstrated that flavonoids, a group of secondary metabolites, play an important role in host plant responses to abiotic stressors (59, 60). Our transcriptome analysis indicated that all the genes associated with flavonoid metabolism were upregulated in plants inoculated with KLBMP 5180 (Fig. S3). Indeed, inoculated plants exhibited higher accumulations of total flavonoids in the leaves (Fig. 4a), and this was consistent with our transcriptomic results. Therefore, KLBMP 5180 might modulate the biosynthesis of these two important secondary metabolites in L. sinense, and this interaction might be associated with NaCl tolerance. This is consistent with previous studies of soybeans and Arabidopsis inoculated with Sinorhizobium meliloti 1021 and Bacillus amyloliquefaciens FZEB42, respectively, and treated with salt (52, 61).
The reduction of Na+ accumulation and the promotion of K+ influx are important for plant salt tolerance (62). Here, the K+ transporter and K+ channel GORK genes were significantly upregulated in inoculated plants treated with 250 mM NaCl, while the sodium/potassium ion P-ATPase family transporter gene was upregulated in inoculated plants treated with 0 mM NaCl (Table S7). In both cases, the accumulation of Na+ was reduced and uptake of K+ was increased. GORK and sodium/potassium ion P-ATPase family transporter genes are critical to the salt stress tolerance of plants, because they regulate the Na+/K+ homeostasis (63). Our results are consistent with previous studies indicating that the K+ transporter gene was significantly upregulated by Bacillus amyloliquefaciens FZEB42 and Bacillus amyloliquefaciens SQR9 in plant roots under NaCl stress (51, 61).
Transcription factors (TFs) may also play key roles in plant salinity tolerance. High percentages of ERF, WRKY, and bHLH TFs were detected in L. sinense seedlings inoculated with KLBMP 5180 (Table S7). ERF TFs are involved in the ethylene (ET) signaling pathway, and many of these TFs have been shown to confer abiotic stress tolerance (64, 65). Similarly, the inoculation of oat seedlings with rhizosphere Klebsiella sp. strain IG 3 positively modulated the expression profile of WRKY1 genes under salt stress (66). The transcription of several bHLH TFs was also increased in oilseed rape leaves under NaCl stress (67).
Trehalose and proline are known osmoprotectants that act as antioxidants under NaCl stress. The expression of the trehalose biosynthetic gene encoding trehalose 6-phosphate phosphatase (TPP) was upregulated in inoculated plants treated with NaCl. Interestingly, consistent with the increase in the proline concentration we observed, the key genes encoding pyrroline-5-carboxylate synthetase (for proline biosynthesis) were only upregulated in salt-treated inoculated seedlings (Table S7). Other bacteria, such as halotolerant actinobacterium Dietzia natronolimnaea STR1 and Arthrobacter sp. strain SU18, also increased proline content in salt-stressed wheat after inoculation (68, 69). Our results indicated that the accumulation of osmoprotectants was modulated by KLBMP 5180 and improved the osmotic adjustment of the host plant under NaCl stress.
Plant hormones are also critical for the adaption of plants to abiotic stressors (70). The expression of auxin-related genes was altered after KLBMP 5180 inoculation, especially in plants not treated with NaCl. Notably, the flavin-containing monooxygenase and indole-3-pyruvate monooxygenase YUCCA8 genes involved in the tryptophan-dependent IAA biosynthesis pathway and response to abiotic tolerance in Arabidopsis (71) were upregulated here. Gibberellin (GA) plays an important role in plant growth and is mediated by the receptor GID1; GID1 is important for seed development and stem growth (72). In plants inoculated with KLBMP 5180 and not exposed to salt, both GID1 and gibberellin 2-beta-dioxygenase genes were upregulated; these genes are key for GA biosynthesis (Table S7). We therefore speculated that the PGP effects modulated by KLBMP 5180 in plants grown without salt were mainly due to changes in the regulation of plant hormones. This is different from the mechanism of mainly regulating the expression of genes related to amino acid transport, metabolism, and secondary metabolite biosynthesis by KLBMP 5180 under salt stress. This also indicates that strain KLBMP 5180 could potentially be used as a plant growth promotion agent to improve the growth of other plants. Interestingly, 1-aminocyclopropane-1-carboxylic acid (ACC) oxidase, an important ethylene (ET) synthesis enzyme that converts ACC to ET, was downregulated in all inoculated seedlings compared to that in noninoculated seedlings. This downregulation might lead to a reduction in ET biosynthesis. Consistent with this result, several ET-responsive TFs were also downregulated in inoculated seedlings not treated with salt. These findings are congruent with a study of salt-treated barley leaves colonized by the root endophyte Piriformospora indica (73).
In conclusion, we characterized the diversity and composition of the endophytic and rhizosphere bacteria associated with the coastal halophyte L. sinense using both culture-dependent and high-throughput sequencing methods. We demonstrated the complexity of the bacterial community and showed that the community composition varied with the habitat (bulk soil, rhizosphere soil, roots, or leaves). We found that the Glutamicibacter halophytocola strain KLBMP 5180 might play an important ecological function, conferring increased growth and salt tolerance on host plants through complex physiological and molecular mechanisms. The mechanism by which L. sinense recruits specific halotolerant PGP bacteria is an interesting area for further study. We have here provided valuable information regarding the interactions between a coastal halophyte and its habitat-adapted symbiotic bacteria. Glutamicibacter strains are promising candidates for PGP agents and for the phytoremediation and eventual cultivation of saline soils in the future.
MATERIALS AND METHODS
Sample collection and bacterial isolation.
We collected three healthy specimens of L. sinense growing in coastal salt marshes in Jiangsu Province, China (119°15′E, 34°45′N), in 2014. The soil pH and NaCl concentration measured were pH 7.9 and 1.56% (wt/wt). The samples were collected with attached rhizosphere soils (adhering to the roots) and bulk soils (nonrhizosphere, 30 cm away from rhizosphere soils). We used sterile spades and gloves for collection. The samples were brought to the laboratory within 24 h and stored at −80°C immediately before the total DNA extraction and at 4°C before the bacterial isolation. The DNA extraction and bacterial isolation were carried out within 5 days.
To isolate endophytic bacteria, we first sterilized the surfaces of the roots and leaves according to a previously published five-step method (74). To confirm surface sterilization, water from the final rinse was spread onto Luria-Bertani (LB) agar medium and incubated at 28°C and 37°C for 5 days. Surface-sterilized roots and leaves were transferred to mortars and crushed thoroughly, and the serial dilutions were prepared (10−1 to 10−3). A total of 80 μl of each dilution was spread on five isolation media: LB agar, nutrient agar (NA), TWYE agar (75), humic acid agar (29), and trehalose-proline agar (76). All isolation media were supplemented with 3% (wt/vol) NaCl and 50 mg/liter nystatin as antifungal agents. We also isolated bacteria from the rhizosphere soil samples with the same dilution plate method described.
Identification of cultivated bacteria.
We examined the morphological characteristics of the bacterial isolates, specifically, the properties of the colonies, the color of the actinomycetes' mycelia, and the diffusible pigments produced. After that, representative isolates were chosen for 16S rRNA gene sequencing and phylogenetic analysis. We extracted genomic DNA from the isolates following the procedures of Li et al. (77). We amplified 16S rRNA using the universal primers 27f (5′-GAGTTTGATCACTGGCTCAG-3′) and 1492r (5′-TACGGCTACCTTGTTACGACTT-3′) according to the methods reported by Qin et al. (78). The PCR products were sequenced by Sangon Biotech (China). We aligned the generated sequences against the EzTaxon-e database (79). The phylogenetic tree was constructed with MEGA 6.0 software (80).
DNA extraction, high-throughput sequencing, and analysis.
The above soil samples and surface-sterilized plant tissues were frozen in liquid nitrogen and then ground to a fine powder. We pooled three samples of each type and then extracted DNA from 0.5 g of powder with a MoBio PowerSoil DNA extraction kit (MoBio Laboratories, Carlsbad, CA, USA) according to the manufacturer's instructions. We amplified bacterial 16S rRNA genes with V5 to V7 region primers 799F (5′-AACMGGATTAGATACCCKG-3′) (81) and 1115R (5′-AGGGTTGCGCTCGTTG-3′) (82), because they were designed to eliminate chloroplast DNA amplification and have been applied successfully in studies of plant bacterial microbiota (83). Sequences were barcoded with an eight-base sequence unique to each sample. Each 20-μl PCR mixture contained 4 μl 5× FastPfu buffer, 0.4 μl FastPfu polymerase, 2 μl deoxynucleoside triphosphates (dNTPs; 2.5 mM), 0.8 μl forward primer (5 μM), 0.8 μl reverse primer (5 μM), and 10 ng template DNA. The PCR conditions were an initial denaturation at 95°C for 3 min, 27 cycles of 95°C for 30 s, 55°C for 30 s, and 72°C for 45 s, a final extension of 72°C for 10 min, and incubation at 4°C. The amplicons were extracted from 2% agarose gels and purified using the AxyPrep DNA gel extraction kit (Axygen Biosciences, Union City, CA, USA) according to the manufacturer's instructions. The purified amplicons were quantified with QuantiFluor-ST (Promega, USA) and then pooled in equimolar aliquots and paired-end sequenced (2 × 250) on an Illumina MiSeq platform at Majorbio Biotech Co., Ltd. (Shanghai, China) according to standard protocols.
We demultiplexed and quality filtered the raw FASTQ files using QIIME (84). Operational taxonomic units (OTUs) were clustered using a 97% similarity cutoff with UPARSE v7.1 (85). We identified and removed chimeric sequences with UCHIME (86). The phylogenetic affiliation of each 16S rRNA gene sequence was analyzed by RDP Classifier (87) against the SILVA 16S rRNA database (88) using a confidence threshold of 70%. All samples were normalized by subsampling to 18,613 sequences, the size of the smallest sample. We used the Mothur program (89) to calculate the alpha diversity and species richness (Chao, Shannon index, Simpson index, and coverage) of the samples at the 97% threshold from normalized data.
Determination of PGP properties of Glutamicibacter strains.
We determined the phosphate solubilization ability by measuring the size of the colony and of the clean zone for bacteria grown on Ca3(PO4)2 agar medium according to the methods described by Franco-Correaa et al. (90). We measured indole-3-acetic acid (IAA) production by growing strains in minimal salt medium (76) supplemented with 0.5 mg · ml−1 tryptophan and then using Salkowski's reagent test. We tested the putative nitrogen-fixing ability of the bacterial strains by incubating them at 28°C for 7 days on nitrogen-free semisolid JNFb medium and then reinoculating the strains in nitrogen-free semisolid medium five times according to the procedure described by Döbereiner et al. (91). We measured ACCD production using a previously described method (92). The ability of isolates to grow at different NaCl concentrations (0 to 2,550 mM, at intervals of 170 mM) and pHs was assessed by growing the strains on ISP 2 basal medium.
Strain inoculations and pot experiments.
We randomly selected three isolates (KLBMP 5180, KLBMP 5252, and KLBMP 5285) of the most common bacterial genus identified (Glutamicibacter) and evaluated their PGP properties under NaCl stress conditions. L. sinense seeds were surface sterilized as previously described (93). Five sets of three surface-sterilized seeds were inoculated with actinobacterial suspensions (1.0 × 107 CFU/ml) using a previously described protocol (94) and placed onto Murashige and Skoog (MS) medium (supplemented with 85 mM NaCl, pH 5.8). We soaked surface-sterilized seeds in sterile water as a control. All germinated seedlings were grown in a greenhouse at 25°C with a 16-h/8-h day/night regime. After 8 weeks, we evaluated the growth of all seedlings.
We then selected the endophytic strain KLBMP 5180 for further evaluation of PGP ability under NaCl stress. Surface-sterilized seeds were sown in plastic pots filled with sterilized vermiculite and soil mix. After germination, one seedling per pot was transplanted to a larger pot (diameter, 9 cm; depth, 12 cm) filled with 200 g sterilized soil. After an additional 4 days of growth, the seedlings were inoculated with either 15 ml strain suspension culture (1.0 × 107 CFU/ml) prepared as described by Qin et al. (94) or 15 ml sterile water (as controls). The inoculations were repeated every 3 days for a total of 5 inoculations. Three days after the last inoculation, the seedlings were irrigated with sterile water containing different concentrations of NaCl (0, 100, or 250 mM), with final soil NaCl concentrations of 0%, 1%, and 1.8% (wt/wt), respectively, and transferred to a greenhouse (25°C with a 16-h/8-h day/night regime). Each treatment was performed on five sets of seedlings. After 21 days, the plants were harvested and the physiological properties and transcriptomes of those treated with 0 and 250 mM NaCl were analyzed.
Plant physiological parameter analysis.
We measured total chlorophyll and proline contents in the plant leaves using the methods described by Ali et al. (95) and Bates et al. (96), respectively. Total flavonoid concentration was measured using the method described by Tavares et al. (97). We measured malondialdehyde (MDA), peroxidase (POD), catalase (CAT), and phenylalanine ammonia-lyase (PAL) in the leaves with dedicated detection kits (Nanjing Jiancheng Bioengineering Institute, China) according to the manufacturer's instructions. We measured Na+, K+, and Ca2+ ions in the leaves with inductively coupled plasma atomic emission spectrometry (ICP-AES; Optima 8000; PerkinElmer Co., USA) according to the methods described by Niu et al. (54).
Transcriptome analysis.
We used TRIzol reagents (Invitrogen, USA) to extract total RNA from the leaves of four sets of seedlings (three biological repeats per set): inoculated, treated with 0 mM NaCl; inoculated, treated with 250 mM NaCl; noninoculated, treated with 0 mM NaCl; and noninoculated, treated with 250 mM NaCl. We assessed the concentration and quality of total RNA with a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, USA) and an Agilent 2100 Bioanalyzer (Agilent Technologies, USA). We used magnetic beads with oligo(dT) to enrich mRNA from total RNA. We then synthesized cDNA, performed end repair, and added single A (adenine) nucleotides at the 3′ ends as described by Zhang et al. (98). Sequencing of the 12 cDNA libraries was performed with an Illumina HiSeq 2500 platform by Biomarker Technology Co. (Beijing, China). The sequencing raw data were processed by removing adaptor sequences and low-quality reads (more than 10% unknown nucleotides and Q30 threshold lower than 85%) and then assembled using Trinity software (99).
Gene expression was calculated as fragments per kilobase of transcript per million fragments mapped (FPKM) using Cufflinks software (100). Differentially expressed genes (DEGs) were identified using the DESeq package (101). Genes with a false discovery rate (FDR) of <0.01 and a log2 ratio of >∣1∣ were defined as DEGs. All mapped unigenes or DEGs were compared and annotated with databases of NCBI nonredundant protein (NR), the Clusters of Orthologous Groups of proteins (COG), Gene Ontology (GO), Kyoto Encyclopedia of Genes and Genomes (KEGG), reviewed protein sequence (Swiss-Prot), and protein families (Pfam) using BLASTX.
Reverse transcription quantitative PCR (RT-qPCR) analysis.
To evaluate RNA-seq gene expression patterns, we performed qPCR using the total RNA samples derived above. The primers used are shown in Table S2 in the supplemental material. First strand cDNA was synthesized from 2.0 μg total RNA with a Probegene MM061 RT reagent kit (Probegene, Inc., Xuzhou, China). We performed RT-qPCR on an Mx3000P system (Applied Biosystems, USA) using 2× SYBR green qPCR master mix (Probegene MQ051) according to the manufacturer's instructions. qPCR cycling conditions were 10 min at 95°C followed by 40 cycles at 95°C for 15 s and 60°C for 30 s. The amplicons were then heated from 60 to 95°C to obtain the melting curve. We used the GAPDH (glyceraldehyde 3-phosphate dehydrogenase) gene as the internal control. Three independent biological and technical replicates were performed. The 2−ΔΔCT method (102) was used to calculate relative gene expression.
Statistical analysis.
Data were analyzed using Sigma Plot (v12.0) software and are represented as mean values ± standard deviations (SDs). Significant differences were analyzed with one-way analyses of variance (ANOVAs). We considered a P value of <0.05 as significant.
Accession number(s).
All generated 16S rRNA gene sequences from bacterial isolates have been submitted to GenBank under accession numbers JX993757 to JX993764, JX993766 to JX993782, JX993784, JX993786, JX993787, JX993789 to JX993791, JX993794 to JX993801, JX993804 to JX993806, KT724296, KT724298, KT724299, and MG874752 to MG874755. The accession numbers are listed in Table S1. Illumina MiSeq sequence reads from DNA from all samples have been deposited in the NCBI Sequence Read Archive (SRA; accession no. SRP132443). All clean sequences from cDNA libraries sequenced with the Illumina HiSeq 2500 for the transcriptome analysis have been deposited into the NCBI SRA database (accession no. SRP131531).
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (no. 31370062), the Qing Lan Project of Jiangsu Province (2014), the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (17KJA180004), and the project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
We thank Biomics Biotechnologies Co. Ltd., Beijing, China, for technical support.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.01533-18.
REFERENCES
- 1.Bragina A, Oberauner-Wappis L, Zachow C, Halwachs B, Thallinger GG, Müller H, Berg G. 2014. The Sphagnum microbiome supports bog ecosystem functioning under extreme conditions. Mol Ecol 23:4498–4510. doi: 10.1111/mec.12885. [DOI] [PubMed] [Google Scholar]
- 2.Trognitz F, Hackl E, Widhalm S, Sessitsch A. 2016. The role of plant-microbiome interactions in weed establishment and control. FEMS Microbiol Ecol 92:fiw138. doi: 10.1093/femsec/fiw138. [DOI] [PubMed] [Google Scholar]
- 3.Hartman K, van der Heijden MG, Roussely-Provent V, Walser JC, Schlaeppi K. 2017. Deciphering composition and function of the root microbiome of a legume plant. Microbiome 5:2. doi: 10.1186/s40168-016-0220-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Müller DB, Vogel C, Bai Y, Vorholt JA. 2016. The plant microbiota: systems-level insights and perspectives. Annu Rev Genet 50:211–234. doi: 10.1146/annurev-genet-120215-034952. [DOI] [PubMed] [Google Scholar]
- 5.Qin S, Xing K, Jiang JH, Xu LH, Li WJ. 2011. Biodiversity, bioactive natural products and biotechnological potential of plant-associated endophytic actinobacteria. Appl Microbiol Biotechnol 89:457–473. doi: 10.1007/s00253-010-2923-6. [DOI] [PubMed] [Google Scholar]
- 6.Gopal M, Gupta A. 2016. Microbiome selection could spur next-generation plant breeding strategies. Front Microbiol 7:1971. doi: 10.3389/fmicb.2016.01971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hacquard S, Spaepen S, Garrido-Oter R, Schulze-Lefert P. 2017. Interplay between innate immunity and the plant microbiota. Annu Rev Phytopathol 55:565–589. doi: 10.1146/annurev-phyto-080516-035623. [DOI] [PubMed] [Google Scholar]
- 8.Santos-Medellín C, Edwards J, Liechty Z, Nguyen B, Sundaresan V. 2017. Drought stress results in a compartment-specific restructuring of the rice root-associated microbiomes. mBio 8:e00764-17. doi: 10.1128/mBio.00764-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glick BR. 2014. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol Res 169:30–39. doi: 10.1016/j.micres.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 10.Nadeem SM, Ahmad M, Zahir ZA, Javaid A, Ashraf M. 2014. The role of mycorrhizae and plant growth promoting rhizobacteria (PGPR) in improving crop productivity under stressful environments. Biotechnol Adv 32:429–448. doi: 10.1016/j.biotechadv.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 11.Bakker PAHM, Berendsen RL, Doornbos RF, Wintermans PCA, Pieterse CMJ. 2013. The rhizosphere revisited: root microbiomics. Front Plant Sci 4:165. doi: 10.3389/fpls.2013.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar M, Brader G, Sessitsch A, Mäki A, van Elsas JD, Nissinen R. 2017. Plants assemble species specific bacterial communities from common core taxa in three arcto-alpine climate zones. Front Microbiol 8:12. doi: 10.3389/fmicb.2017.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peiffer JA, Spor A, Koren O, Jin Z, Tringe SG, Dangl JL, Buckler ES, Ley RE. 2013. Diversity and heritability of the maize rhizosphere microbiome under field conditions. Proc Natl Acad Sci U S A 110:6548–6553. doi: 10.1073/pnas.1302837110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edwards J, Johnson C, Santos-Medellín C, Lurie E, Podishetty NK, Bhatnagar S, Eisen JA, Sundaresan V. 2015. Structure, variation, and assembly of the root-associated microbiomes of rice. Proc Natl Acad Sci U S A 112:E911–E920. doi: 10.1073/pnas.1414592112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bulgarelli D, Garrido-Oter R, Münch PC, Weiman A, Dröge J, Pan Y, McHardy AC, Schulze-Lefert P. 2015. Structure and function of the bacterial root microbiota in wild and domesticated barley. Cell Host Microbe 17:392–403. doi: 10.1016/j.chom.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Epstein E, Bloom AJ. 2005. Mineral nutrition of plants: principles and perspectives, 2nd ed, p 342–351. Sinauer Association, Sunderland, MA. [Google Scholar]
- 17.Gilbert M, Pammenter N, Ripley B. 2008. The growth responses of coastal dune species are determined by nutrient limitation and sand burial. Oecologia 156:169–178. doi: 10.1007/s00442-008-0968-3. [DOI] [PubMed] [Google Scholar]
- 18.Deinlein U, Stephan AB, Horie T, Luo W, Xu G, Schroeder JI. 2014. Plant salt-tolerance mechanisms. Trends Plant Sci 19:371–379. doi: 10.1016/j.tplants.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong BH. 2005. Research on the conservation of Limonium sinense in the coast of Jiangsu, China. Wild Plant Res 24:28–30. [Google Scholar]
- 20.Tang XH, Yan LF, Gao J, Yang XL, Xu YX, Ge HY, Yang HD. 2012. Antitumor and immunomodulatory activity of polysaccharides from the root of Limonium sinense Kuntze. Int J Biol Macromol 51:1134–1139. doi: 10.1016/j.ijbiomac.2012.08.037. [DOI] [PubMed] [Google Scholar]
- 21.Hsu WC, Chang SP, Lin LC, Li CL, Richardson CD, Lin CC, Lin LT. 2015. Limonium sinense and gallic acid suppress hepatitis C virus infection by blocking early viral entry. Antiviral Res 118:139–147. doi: 10.1016/j.antiviral.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 22.Rodriguez RJ, Henson J, Van Volkenburgh E, Hoy M, Wright L, Beckwith F, Kim YO, Redman RS. 2008. Stress tolerance in plants via habitat-adapted symbiosis. ISME J 2:404–416. doi: 10.1038/ismej.2007.106. [DOI] [PubMed] [Google Scholar]
- 23.Redman RS, Kim YO, Woodward CJ, Greer C, Espino L, Doty SL, Rodriguez RJ. 2011. Increased fitness of rice plants to abiotic stress via habitat adapted symbiosis: a strategy for mitigating impacts of climate change. PLoS One 6:e14823. doi: 10.1371/journal.pone.0014823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lau JA, Lennon JT. 2012. Rapid responses of soil microorganisms improve plant fitness in novel environments. Proc Natl Acad Sci U S A 109:14058–14062. doi: 10.1073/pnas.1202319109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sgroy V, Cassán F, Masciarelli O, Del Papa MF, Lagares A, Luna V. 2009. Isolation and characterization of endophytic plant growth-promoting (PGPB) or stress homeostasis-regulating (PSHB) bacteria associated to the halophyte Prosopis strombulifera. Appl Microbiol Biotechnol 85:371–381. doi: 10.1007/s00253-009-2116-3. [DOI] [PubMed] [Google Scholar]
- 26.Marasco R, Mapelli F, Rolli E, Mosqueira MJ, Fusi M, Bariselli P, Reddy M, Cherif A, Tsiamis G, Borin S, Daffonchio D. 2016. Salicornia strobilacea (synonym of Halocnemum strobilaceum) grown under different tidal regimes selects rhizosphere bacteria capable of promoting plant growth. Front Microbiol 7:1286. doi: 10.3389/fmicb.2016.01286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qin Y, Druzhinina IS, Pan X, Yuan Z. 2016. Microbially mediated plant salt tolerance and microbiome-based solutions for saline agriculture. Biotechnol Adv 34:1245–1259. doi: 10.1016/j.biotechadv.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 28.Zhao S, Zhou N, Zhao ZY, Zhang K, Wu GH, Tian CY. 2016. Isolation of endophytic plant growth-promoting bacteria associated with the halophyte Salicornia europaea and evaluation of their promoting activity under salt stress. Curr Microbiol 73:574–581. doi: 10.1007/s00284-016-1096-7. [DOI] [PubMed] [Google Scholar]
- 29.Feng WW, Wang TT, Bai JL, Ding P, Xing K, Jiang JH, Peng X, Qin S. 2017. Glutamicibacter halophytocola sp. nov., an endophytic actinomycete isolated from the roots of a coastal halophyte, Limonium sinense. Int J Syst Evol Microbiol 67:1120–1125. doi: 10.1099/ijsem.0.001775. [DOI] [PubMed] [Google Scholar]
- 30.Wang XM, Dong J, Liu Y, Gao HW. 2010. A novel dehydration-responsive element-binding protein from Caragana korshinskii is involved in the response to multiple abiotic stresses and enhances stress tolerance in transgenic tobacco. Plant Mol Biol Rep 28:664–675. doi: 10.1007/s11105-010-0196-y. [DOI] [Google Scholar]
- 31.Agarwal PK, Gupta K, Lopato S, Agarwal P. 2017. Dehydration responsive element binding transcription factors and their applications for the engineering of stress tolerance. J Exp Bot 68:2135–2148. doi: 10.1093/jxb/erx118. [DOI] [PubMed] [Google Scholar]
- 32.Vandenkoornhuyse P, Quaiser A, Duhamel M, Le Van A, Dufresne A. 2015. The importance of the microbiome of the plant holobiont. New Phytol 206:1196–1206. doi: 10.1111/nph.13312. [DOI] [PubMed] [Google Scholar]
- 33.Lundberg DS, Lebeis SL, Paredes SH, Yourstone S, Gehring J, Malfatti S, Tremblay J, Engelbrektson A, Kunin V, Del Rio TG, Edgar RC, Eickhorst T, Ley RE, Hugenholtz P, Tringe SG, Dangl JL. 2012. Defining the core Arabidopsis thaliana root microbiome. Nature 488:86–90. doi: 10.1038/nature11237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beckers B, Op De Beeck M, Weyens N, Van Acker R, Van Montagu M, Boerjan W, Vangronsveld J. 2016. Lignin engineering in field-grown poplar trees affects the endosphere bacterial microbiome. Proc Natl Acad Sci U S A 113:2312–2317. doi: 10.1073/pnas.1523264113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Niu B, Paulson JN, Zheng X, Kolter R. 2017. Simplified and representative bacterial community of maize roots. Proc Natl Acad Sci U S A 114:E2450–E2459. doi: 10.1073/pnas.1616148114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barnawal D, Bharti N, Maji D, Chanotiya CS, Kalra A. 2014. ACC deaminase-containing Arthrobacter protophormiae induces NaCl stress tolerance through reduced ACC oxidase activity and ethylene production resulting in improved nodulation and mycorrhization in Pisum sativum. J Plant Physiol 171:884–894. doi: 10.1016/j.jplph.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 37.Palaniyandi SA, Damodharan K, Yang SH, Suh JW. 2014. Streptomyces sp. strain PGPA 39 alleviates salt stress and promotes growth of “Micro Tom” tomato plants. J Appl Microbiol 117:766–773. doi: 10.1111/jam.12563. [DOI] [PubMed] [Google Scholar]
- 38.Fan P, Chen D, He Y, Zhou Q, Tian Y, Gao L. 2016. Alleviating salt stress in tomato seedlings using Arthrobacter and Bacillus megaterium isolated from the rhizosphere of wild plants grown on saline-alkaline lands. Int J Phytoremediation 18:1113–1121. doi: 10.1080/15226514.2016.1183583. [DOI] [PubMed] [Google Scholar]
- 39.Wang M, Yang P, Falcão Salles J. 2016. Distribution of root-associated bacterial communities along a salt-marsh primary succession. Front Plant Sci 6:1188. doi: 10.3389/fpls.2015.01188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tian XY, Zhang CS. 2017. Illumina-based analysis of endophytic and rhizosphere bacterial diversity of the coastal halophyte Messerschmidia sibirica. Front Microbiol 8:2288. doi: 10.3389/fmicb.2017.02288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beauchemin NJ, Furnholm T, Lavenus J, Svistoonoff S, Doumas P, Bogusz D, Laplaze L, Tisa LS. 2012. Casuarina root exudates alter the physiology, surface properties, and plant infectivity of Frankia sp. strain CcI3. Appl Environ Microbiol 78:575–580. doi: 10.1128/AEM.06183-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mora-Ruiz MR, Font-Verdera F, Díaz-Gil C, Urdiain M, Rodríguez-Valdecantos G, González B, Orfila A, Rosselló-Móra R. 2015. Moderate halophilic bacteria colonizing the phylloplane of halophytes of the subfamily Salicornioideae (Amaranthaceae). Syst Appl Microbiol 38:406–416. doi: 10.1016/j.syapm.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 43.Shi YW, Lou K, Li C, Wang L, Zhao ZY, Zhao S, Tian CY. 2015. Illumina based analysis of bacterial diversity related to halophytes Salicornia europaea and Sueada aralocaspica. J Microbiol 53:678–685. doi: 10.1007/s12275-015-5080-x. [DOI] [PubMed] [Google Scholar]
- 44.Long HH, Sonntag DG, Schmidt DD, Baldwin IT. 2010. The structure of the culturable root bacterial endophyte community of Nicotiana attenuata is organized by soil composition and host plant ethylene production and perception. New Phytol 185:554–567. doi: 10.1111/j.1469-8137.2009.03079.x. [DOI] [PubMed] [Google Scholar]
- 45.Busse HJ. 2016. Review of the taxonomy of the genus Arthrobacter, emendation of the genus Arthrobacter sensu lato, proposal to reclassify selected species of the genus Arthrobacter in the novel genera Glutamicibacter gen. nov., Paeniglutamicibacter gen. nov., Pseudoglutamicibacter gen. nov., Paenarthrobacter gen. nov. and Pseudarthrobacter gen. nov., and emended description of Arthrobacter roseus. Int J Syst Evol Microbiol 66:9–37. doi: 10.1099/ijsem.0.000702. [DOI] [PubMed] [Google Scholar]
- 46.Ullah S, Bano A. 2015. Isolation of plant-growth-promoting rhizobacteria from rhizospheric soil of halophytes and their impact on maize (Zea mays L.) under induced soil salinity. Can J Microbiol 61:307–313. doi: 10.1139/cjm-2014-0668. [DOI] [PubMed] [Google Scholar]
- 47.Barnawal D, Bharti N, Pandey SS, Pandey A, Chanotiya CS, Kalra A. 2017. Plant growth-promoting rhizobacteria enhance wheat salt and drought stress tolerance by altering endogenous phytohormone levels and TaCTR1/TaDREB2 expression. Physiol Plant 161:502–514. doi: 10.1111/ppl.12614. [DOI] [PubMed] [Google Scholar]
- 48.Fernández-González AJ, Martínez-Hidalgo P, Cobo-Díaz JF, Villadas PJ, Martínez-Molina E, Toro N, Tringe SG, Fernández-López M. 2017. The rhizosphere microbiome of burned holm-oak: potential role of the genus Arthrobacter in the recovery of burned soils. Sci Rep 7:6008. doi: 10.1038/s41598-017-06112-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yuan Z, Druzhinina IS, Labbé J, Redman R, Qin Y, Rodriguez R, Zhang C, Tuskan GA, Lin F. 2016. Specialized microbiome of a halophyte and its role in helping non-host plants to withstand salinity. Sci Rep 6:32467. doi: 10.1038/srep32467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Akram MS, Shahid M, Tariq M, Azeem M, Javed MT, Saleem S, Riaz S. 2016. Deciphering Staphylococcus sciuri SAT-17 mediated anti-oxidative defense mechanisms and growth modulations in salt stressed maize (Zea mays L.). Front Microbiol 7:867. doi: 10.3389/fmicb.2016.00867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen L, Liu Y, Wu G, Veronican Njeri K, Shen Q, Zhang N, Zhang R. 2016. Induced maize salt tolerance by rhizosphere inoculation of Bacillus amyloliquefaciens SQR9. Physiol Plant 158:34–44. doi: 10.1111/ppl.12441. [DOI] [PubMed] [Google Scholar]
- 52.Qu L, Huang Y, Zhu C, Zeng H, Shen C, Liu C, Zhao Y, Pi E. 2016. Rhizobia-inoculation enhances the soybean's tolerance to salt stress. Plant Soil 400:209–222. doi: 10.1007/s11104-015-2728-6. [DOI] [Google Scholar]
- 53.Han QQ, Lü XP, Bai JP, Qiao Y, Paré PW, Wang SM, Zhang JL, Wu YN, Pang XP, Xu WB, Wang ZL. 2014. Beneficial soil bacterium Bacillus subtilis (GB03) augments salt tolerance of white clover. Front Plant Sci 5:525. doi: 10.3389/fpls.2014.00525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Niu SQ, Li HR, Paré PW, Aziz M, Wang SM, Shi H, Li J, Han QQ, Guo SQ, Li J, Guo Q, Ma Q, Zhang JL. 2016. Induced growth promotion and higher salt tolerance in the halophyte grass Puccinellia tenuiflora by beneficial rhizobacteria. Plant Soil 407:217–230. doi: 10.1007/s11104-015-2767-z. [DOI] [Google Scholar]
- 55.Zhang JL, Aziz M, Qiao Y, Han QQ, Li J, Wang YQ, Shen X, Wang SM, Paié PW. 2014. Soil microbe Bacillus subtilis (GB03) induces biomass accumulation and salt tolerance with lower sodium accumulation in wheat. Crop Pasture Sci 65:423–427. doi: 10.1071/CP13456. [DOI] [Google Scholar]
- 56.Wu P, Guo QQ, Qin ZW. 2016. The fungicide propamocarb increases lignin by activating the phenylpropanoid pathway in Cucumis sativus L. Hortic Environ Biotechnol 57:511–518. doi: 10.1007/s13580-016-0049-1. [DOI] [Google Scholar]
- 57.Chen T, Cai X, Wu X, Karahara I, Schreiber L, Lin J. 2011. Casparian strip development and its potential function in salt tolerance. Plant Signal Behav 6:1499–1502. doi: 10.4161/psb.6.10.17054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.de Lima RB, dos Santos TB, Vieira LG, de Lourdes Lúcio Ferrarese M, Ferrarese-Filho O, Donatti L, Boeger MR, de Oliveira Petkowicz CL. 2014. Salt stress alters the cell wall polysaccharides and anatomy of coffee (Coffea arabica L.) leaf cells. Carbohydr Polym 112:686–694. doi: 10.1016/j.carbpol.2014.06.042. [DOI] [PubMed] [Google Scholar]
- 59.Nakabayashi R, Yonekura-Sakakibara K, Urano K, Suzuki M, Yamada Y, Nishizawa T, Matsuda F, Kojima M, Sakakibara H, Shinozaki K, Michael AJ, Tohge T, Yamazaki M, Saito K. 2014. Enhancement of oxidative and drought tolerance in Arabidopsis by overaccumulation of antioxidant flavonoids. Plant J 77:367–379. doi: 10.1111/tpj.12388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhao GM, Han Y, Sun X, Li SH, Shi QM, Wang CH. 2015. Salinity stress increases secondary metabolites and enzyme activity in safflower. Ind Crops Prod 64:175–181. doi: 10.1016/j.indcrop.2014.10.058. [DOI] [Google Scholar]
- 61.Liu S, Hao H, Lu X, Zhao X, Wang Y, Zhang Y, Xie Z, Wang R. 2017. Transcriptome profiling of genes involved in induced systemic salt tolerance conferred by Bacillus amyloliquefaciens FZB42 in Arabidopsis thaliana. Sci Rep 7:10795. doi: 10.1038/s41598-017-11308-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shabala S, Cuin TA. 2008. Potassium transport and salt tolerance. Physiol Plant 133:651–669. doi: 10.1111/j.1399-3054.2007.01008.x. [DOI] [PubMed] [Google Scholar]
- 63.Assaha DVM, Ueda A, Saneoka H, Al-Yahyai R, Yaish MW. 2017. The role of Na+ and K+ transporters in salt stress adaptation in glycophytes. Front Physiol 8:509. doi: 10.3389/fphys.2017.00509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nakano T, Suzuki K, Fujimura T, Shinshi H. 2006. Genome-wide analysis of the ERF gene family in Arabidopsis and rice. Plant Physiol 140:411–432. doi: 10.1104/pp.105.073783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hu L, Liu S. 2011. Genome-wide identification and phylogenetic analysis of the ERF gene family in cucumbers. Genet Mol Biol 34:624–633. doi: 10.1590/S1415-47572011005000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sapre S, Gontia-Mishra I, Tiwari S. 2018. Klebsiella sp. confers enhanced tolerance to salinity and plant growth promotion in oat seedlings (Avena sativa). Microbiol Res 206:25–32. doi: 10.1016/j.micres.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 67.Yong HY, Zou Z, Kok EP, Kwan BH, Chow K, Nasu S, Nanzyo M, Kitashiba H, Nishio T. 2014. Comparative transcriptome analysis of leaves and roots in response to sudden increase in salinity in Brassica napus by RNA-seq. Biomed Res Int 2014:467395. doi: 10.1155/2014/467395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Upadhyay SK, Singh DP. 2015. Effect of salt-tolerant plant growth-promoting rhizobacteria on wheat plants and soil health in a saline environment. Plant Biol (Stuttg) 17:288–293. doi: 10.1111/plb.12173. [DOI] [PubMed] [Google Scholar]
- 69.Bharti N, Pandey SS, Barnawal D, Patel VK, Kalra A. 2016. Plant growth promoting rhizobacteria Dietzia natronolimnaea modulates the expression of stress responsive genes providing protection of wheat from salinity stress. Sci Rep 6:34768. doi: 10.1038/srep34768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Verma V, Ravindran P, Kumar PP. 2016. Plant hormone-mediated regulation of stress responses. BMC Plant Biol 16:86. doi: 10.1186/s12870-016-0771-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cheol Park H, Cha JY, Yun DJ. 2013. Roles of YUCCAs in auxin biosynthesis and drought stress responses in plants. Plant Signal Behav 8:e24495. doi: 10.4161/psb.24495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hauvermale AL, Ariizumi T, Steber CM. 2014. The roles of the GA receptors GID1a, GID1b, and GID1c in sly1-independent GA signaling. Plant Signal Behav 9:e28030. doi: 10.4161/psb.28030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ghaffari MR, Ghabooli M, Khatabi B, Hajirezaei MR, Schweizer P, Salekdeh GH. 2016. Metabolic and transcriptional response of central metabolism affected by root endophytic fungus Piriformospora indica under salinity in barley. Plant Mol Biol 90:699–717. doi: 10.1007/s11103-016-0461-z. [DOI] [PubMed] [Google Scholar]
- 74.Qin S, Li J, Chen HH, Zhao GZ, Zhu WY, Jiang CL, Xu LH, Li WJ. 2009. Isolation, diversity, and antimicrobial activity of rare actinobacteria from medicinal plants of tropical rain forests in Xishuangbanna, China. Appl Environ Microbiol 75:6176–6186. doi: 10.1128/AEM.01034-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Coombs JT, Franco CMM. 2003. Isolation and identification of actinobacteria from surface-sterilized wheat roots. Appl Environ Microbiol 69:5603–5608. doi: 10.1128/AEM.69.9.5603-5608.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Qin S, Miao Q, Feng WW, Wang Y, Zhu X, Xing K, Jiang JH. 2015. Biodiversity and plant growth promoting traits of culturable endophytic actinobacteria associated with Jatropha curcas L. growing in Panxi dry-hot valley soil. Appl Soil Ecol 93:47–55. doi: 10.1016/j.apsoil.2015.04.004. [DOI] [Google Scholar]
- 77.Li WJ, Xu P, Schumann P, Zhang YQ, Pukall R, Xu LH, Stackebrandt E, Jiang CL. 2007. Georgenia ruanii sp. nov., a novel actinobacterium isolated from forest soil in Yunnan (China) and emended description of the genus Georgenia. Int J Syst Evol Microbiol 57:1424–1428. doi: 10.1099/ijs.0.64749-0. [DOI] [PubMed] [Google Scholar]
- 78.Qin S, Chen HH, Zhao GZ, Li J, Zhu WY, Xu LH, Jiang JH, Li WJ. 2012. Abundant and diverse endophytic actinobacteria associated with medicinal plant Maytenus austroyunnanensis in Xishuangbanna tropical rainforest revealed by culture-dependent and culture-independent methods. Environ Microbiol Rep 4:522–531. doi: 10.1111/j.1758-2229.2012.00357.x. [DOI] [PubMed] [Google Scholar]
- 79.Kim OS, Cho YJ, Lee K, Yoon SH, Kim M, Na H, Park SC, Jeon YS, Lee JH, Yi H, Won S, Chun J. 2012. Introducing EzTaxon-e: a prokaryotic 16S rRNA gene sequence database with phylotypes that represent uncultured species. Int J Syst Evol Microbiol 62:716–721. doi: 10.1099/ijs.0.038075-0. [DOI] [PubMed] [Google Scholar]
- 80.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chelius MK, Triplett EW. 2001. The diversity of archaea and bacteria in association with the roots of Zea mays L. Microb Ecol 41:252–263. doi: 10.1007/s002480000087. [DOI] [PubMed] [Google Scholar]
- 82.Reysenbach A, Pace N. 1995. Reliable amplification of hyperthermophilic archaeal 16S rRNA genes by the polymerase chain reaction, p 101–107. In Robb F. (ed), Archaea: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 83.Shade A, McManus PS, Handelsman J. 2013. Unexpected diversity during community succession in the apple flower microbiome. mBio 4:e00602-12. doi: 10.1128/mBio.00602-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R. 2010. QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Edgar RC. 2013. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods 10:996–998. doi: 10.1038/nmeth.2604. [DOI] [PubMed] [Google Scholar]
- 86.Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R. 2011. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27:2194–2200. doi: 10.1093/bioinformatics/btr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang Q, Garrity GM, Tiedje JM, Cole JR. 2007. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 73:5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glöckner FO. 2013. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res 41:D590–D596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, Sahl JW, Stres B, Thallinger GG, Van Horn DJ, Weber CF. 2009. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75:7537–7541. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Franco-Correaa M, Quintanaa A, Duquea C, Suareza C, Rodrígueza MX, Bareab JM. 2010. Evaluation of actinomycete strains for key traits related with plant growth promotion and mycorrhiza helping activities. Appl Soil Ecol 45:209–217. doi: 10.1016/j.apsoil.2010.04.007. [DOI] [Google Scholar]
- 91.Döbereiner HG, Evans E, Seifert U, Wortis M. 1995. Spinodal fluctuations of budding vesicles. Phys Rev Lett 75:3360–3363. doi: 10.1103/PhysRevLett.75.3360. [DOI] [PubMed] [Google Scholar]
- 92.Penrose DM, Glick BR. 2003. Methods for isolating and characterizing ACC deaminase-containing plant growth-promoting rhizobacteria. Physiol Plant 118:10–15. doi: 10.1034/j.1399-3054.2003.00086.x. [DOI] [PubMed] [Google Scholar]
- 93.Qin S, Feng WW, Wang TT, Ding P, Xing K, Jiang JH. 2017. Plant growth-promoting effect and genomic analysis of the beneficial endophyte Streptomyces sp. KLBMP 5084 isolated from halophyte Limonium sinense. Plant Soil 416:117–132. doi: 10.1007/s11104-017-3192-2. [DOI] [Google Scholar]
- 94.Qin S, Zhang YJ, Yuan B, Xu PY, Xing K, Wang J, Jiang JH. 2014. Isolation of ACC deaminase-producing habitat-adapted symbiotic bacteria associated with halophyte Limonium sinense (Girard) Kuntze and evaluating their plant growth-promoting activity under salt stress. Plant Soil 374:753–766. doi: 10.1007/s11104-013-1918-3. [DOI] [Google Scholar]
- 95.Ali S, Charles TC, Glick BR. 2014. Amelioration of high salinity stress damage by plant growth-promoting endophytes that contain ACC deaminase. Plant Physiol Biochem 80:160–167. doi: 10.1016/j.plaphy.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 96.Bates L, Waldren R, Teare I. 1973. Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207. doi: 10.1007/BF00018060. [DOI] [Google Scholar]
- 97.Tavares L, Carrilho D, Tyagi M, Barata D, Serra AT, Duarte CMM, Duarte RO, Feliciano RP, Bronze MR, Chicau P, Espirito-Santo MD, Ferreira RB, dos Santos CN. 2010. Antioxidant capacity of Macaronesian traditional medicinal plants. Molecules 15:2576–2592. doi: 10.3390/molecules15042576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang J, Wang J, Jiang W, Liu J, Yang S, Gai J, Li Y. 2016. Identification and analysis of NaHCO3 stress responsive genes in wild soybean (Glycine soja) roots by RNA-seq. Front Plant Sci 7:1842. doi: 10.3389/fpls.2016.01842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Grabherr MG, Haas BJ, Yassour M, Levin JZ, Thompson DA, Amit I, Adiconis X, Fan L, Raychowdhury R, Zeng Q, Chen Z, Mauceli E, Hacohen N, Gnirke A, Rhind N, di Palma F, Birren BW, Nusbaum C, Lindblad-Toh K, Friedman N, Regev A. 2011. Full-length transcriptome assembly from RNA-seq data without a reference genome. Nat Biotechnol 29:644–652. doi: 10.1038/nbt.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, Salzberg SL, Wold BJ, Pachter L. 2010. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol 28:511–515. doi: 10.1038/nbt.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Anders S, Huber W. 2010. Differential expression analysis for sequence count data. Genome Biol 11:R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.