Abstract
Introduction
We have developed an implant-type tissue-engineered cartilage using a poly-l-lactide scaffold. In a clinical study, it was inserted into subcutaneous areas of nasal dorsum in three patients, to correct cleft lip–nose deformity. The aim of this study was to helping evaluation on the efficacy of the regenerative cartilage.
Methods
3D data of nasal shapes were compared between before and after surgery in computed tomography (CT) images. Morphological and qualitative changes of transplants in the body were also evaluated on MRI, for one year.
Results
The 3D data from CT images showed effective augmentation (>2 mm) of nasal dorsum in almost whole length, observed on the medial line of faces. It was maintained by 1 year post-surgery in all patients, while affected curves of nasal dorsum was not detected throughout the observation period. In magnetic resonance imaging (MRI), the images of transplanted cartilage had been observed until 1 year post-surgery. Those images were seemingly not straight when viewed from the longitudinal plain, and may have shown gentle adaptation to the surrounding nasal bones and alar cartilage tissues.
Conclusion
Those findings suggested the potential efficacy of this cartilage on improvement of cleft lip–nose deformity. A clinical trial is now being performed for industrialization.
Keywords: Tissue engineering, Cartilage, Cleft lip and palate, Nose, Three dimension
Highlights
-
•
Cleft lip–nose deformity was corrected by tissue-engineered cartilage.
-
•
Effective augmentation was observed on the medial line of nasal dorsum for 1 year.
-
•
Images of transplanted cartilage had been detected by MRI until 1 year.
1. Introduction
In regenerative medicine of cartilage, local cartilage defects of the knee and elbow joints have been repaired by autologous cell transplantation. Injection of suspension containing cultured articular chondrocytes suspension has been the main method of autologous chondrocyte transplantation [1], while another option was an application of mixture with collagen hydrogel [2]. In addition, bone marrow-derived [3], synovium-derived [4], and fat-derived [5] mesenchymal stem cells have been used as another cell sources. With those treatments, improvement of symptoms, such as pain, and tissue repair have been reported.
Otherwise, cartilage disease generally manifested variety in severity. It is not limited to local defects of articular cartilage, but also includes facial cartilage defects and hypoplasia that are accompanied by huge cartilage defects. Cleft lip and palate-associated nose deformity is one example. Its symptoms include a tilted low nose and lateral imbalance between the nasal alae [6]. This deformity has been treated by grafting autologous bone, autologous cartilage, or silicone implant into the nasal dorsum and apex. However, those treatments have disadvantages, such as that the nose becomes hard, curved, or exposed [7]. Some difficulty is derived from the point that no ideal material can be obtained among existing transplants. Introduction of innovative treatment, regenerative medicine, has been expected.
Because conventional regenerative cartilage was prepared with cell suspension [1] or mixture with gel [2], and had low mechanical strength, it was hardly applied for correction of cleft lip–nose deformity. We have developed tissue-engineered cartilage with 3D morphology and high mechanical strength using a biodegradable polymer (poly-l-lactide, PLLA) scaffold [8]. We termed it as an implant-type tissue-engineered cartilage. Using this implant-type tissue-engineered cartilage, we corrected nose deformity in cleft lip and palate patients as a clinical study [9]. In this clinical study, no serious adverse events that were associated with the tissue-engineered cartilage occurred throughout the postoperative 3 years. The efficacy was also confirmed by comparing the lateral cephalogram between before and after surgery, in which more than 2-mm augmentation was observed at 3 months after surgery and was maintained for 3 years.
However, only 2D evaluation could be made, when cephalograms were used for quantitative analyses. Since the nose is located in the center of the face and it has a protruding 3D structure, the corrected nose shape should be 3-dimensionally analyzed. Moreover, regarding information on internal properties of regenerative cartilage that used a scaffold material and was transplanted subcutaneously, few studies has been reported in which cartilage regeneration was confirmed in a biopsy specimen from patients [9]. No morphological or biochemical change in the whole transplants of regenerative cartilage has been reported, yet. Although computed tomography (CT) images hardly evaluate biological properties of cartilage in the body, magnetic resonance imaging (MRI) has been frequently used, in which, for example, a contrast agent-based MRI technique, delayed gadolinium-enhanced MR imaging of cartilage (dGEMRIC), reflects glycosaminoglycan content within cartilage [10]. In this study, we traced changes in transplanted regenerative cartilage in the body utilizing MRI images.
Thus, in a small cohort treated with implant-type tissue-engineered cartilage for nose deformity associated with cleft lip and palate (3 patients, 1 year of follow-up period), firstly, 3D data of nasal shapes were prepared from CT images and compared between before and after surgery to analyze 3D changes. Secondly, morphological and qualitative changes of transplants in the body were evaluated on MRI, aiming at helping evaluation on the efficacy of the implant-type regenerated cartilage.
2. Patients and methods
2.1. Patients and procedures
This study conformed with the Declaration of Helsinki. It followed the guidelines for clinical research using human stem cells formulated by the Ministry of Health, Labour and Welfare of Japan, and was approved by the Ethics Committee of the University of Tokyo and Minister of Health, Labour and Welfare of Japan. We recruited three patients with a nasal deformity caused by cleft lip and palate at the University of Tokyo Hospital (Tokyo, Japan). All patients gave written informed consent.
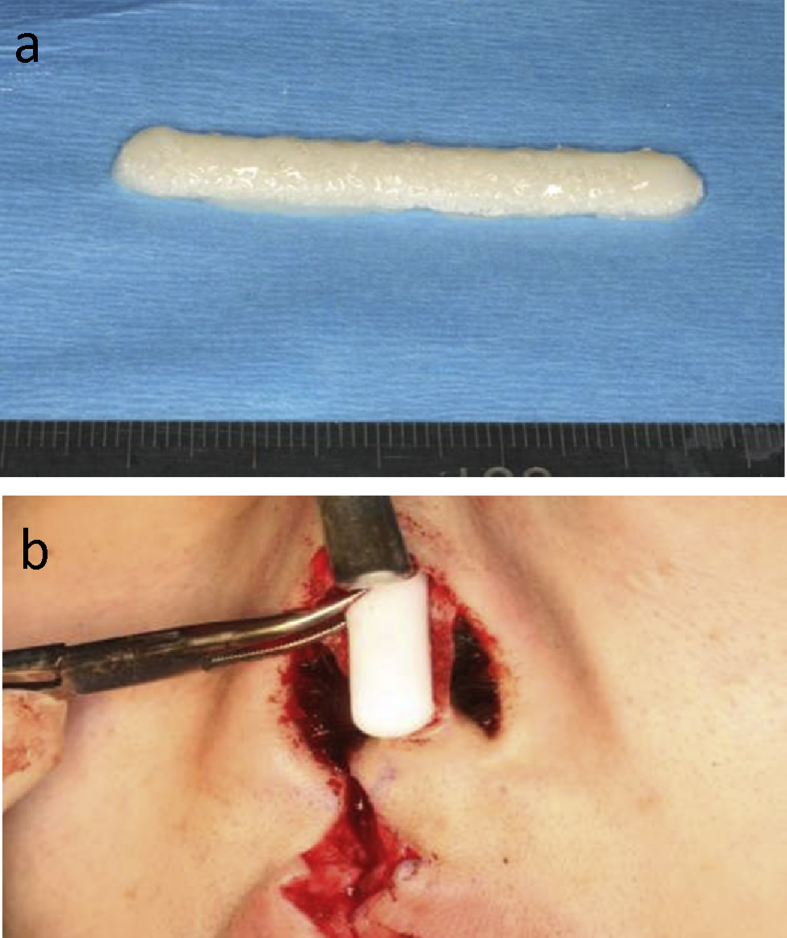
Detailed procedures were described in the previous paper [9]. 390 mL of autologous serum and 0.1 g of auricular cartilage were obtained from a patient. In the cell processing center of the University of Tokyo Hospital, chondrocytes were isolated from the auricular cartilage after collagenase digestion, and were cultured for 4 weeks in the medium containing the autologous serum with insulin and fibroblast growth factor 2 [11]. The chondrocytes were finally expanded to 240 million in cell number. Cultured chondrocytes were mixed with atelocollagen hydrogel (Atelocollagen implant, Koken, Tokyo, Japan) at the density of 108 cells/mL [12], and were then administered into the PLLA scaffold (pore size: approximately 200 μm, porosity: approximately 95%) with the dome-like shape of 50-mm long, 6-mm wide and 3-mm thick (KRI, Kyoto, Japan) [8], to make an implant-type tissue-engineered cartilage (Fig. 1a). All processes were recorded by traceability system.
Fig. 1.
Implant-type tissue-engineered cartilage. The tissue-engineered cartilage was dome-shaped with 5 cm long, 6 mm wide and 3 mm thick (a). It was carefully inserted into the nasal dorsum (b).
In the transplantation of the implant-type tissue-engineered cartilage, the marginal incision of the bilateral nostrils was connected by a transcolumellar incision (gull-in-flight incision). A pocket was dissected over the nasal dorsum through the gull-flight-incision. The open method allowed direct vision of the cartilaginous and bony vault. The periosteum over the nasal bones was raised to enable a transplant to lie in close contact with the bones. The tissue-engineered cartilage was inserted into a subcutaneous pocket formed in the nasal dorsum (Fig. 1b). The curved nasal septum cartilage was removed and retransplanted into columella [13].
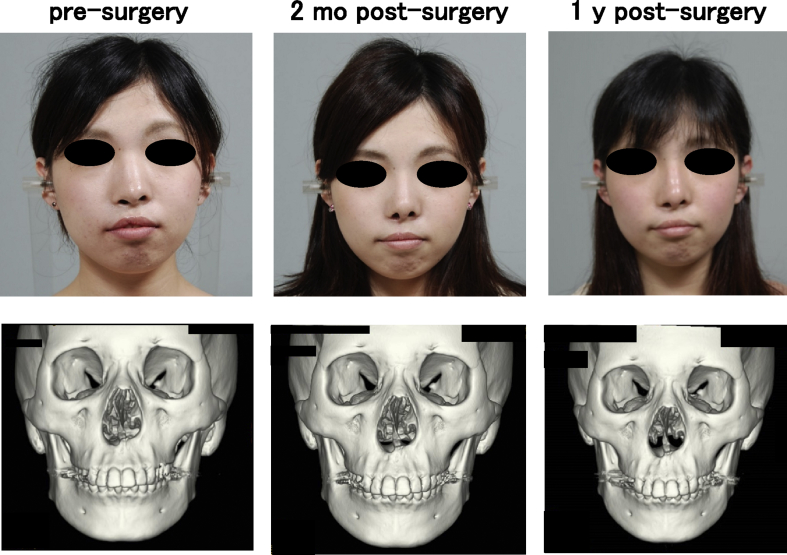
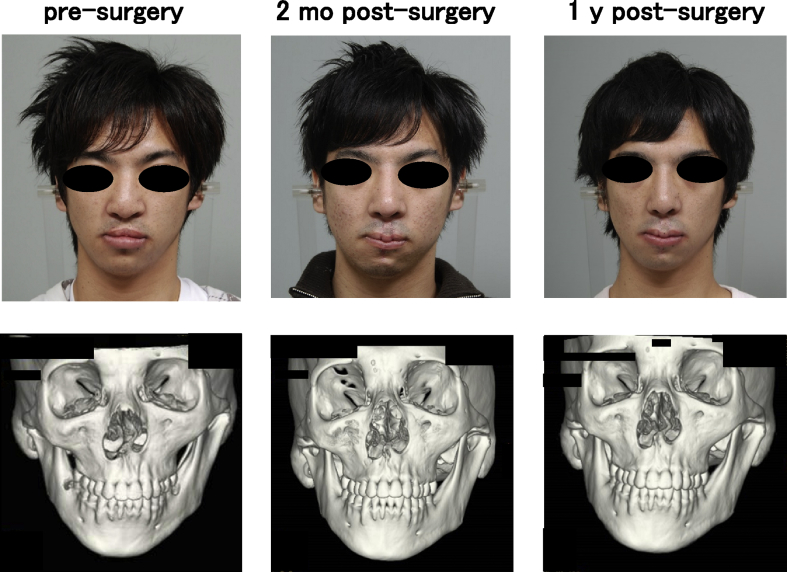
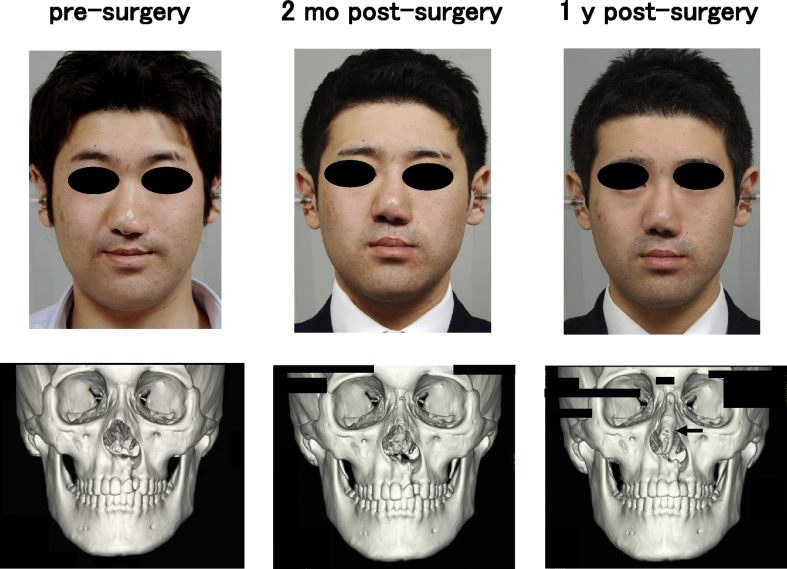
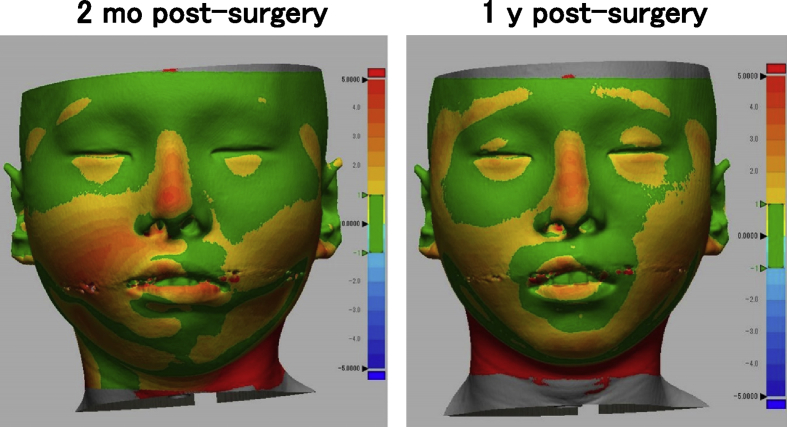
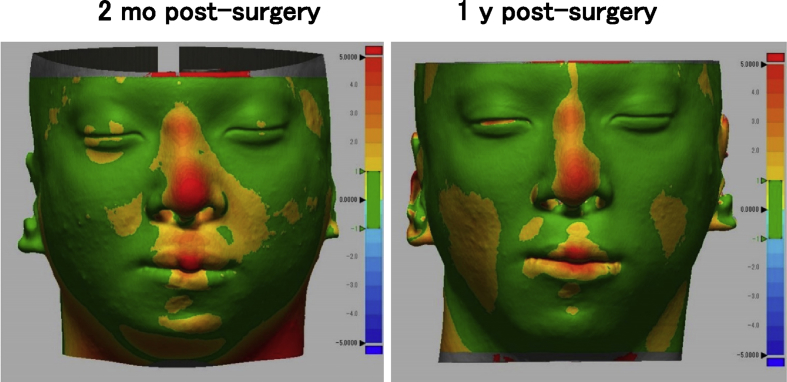
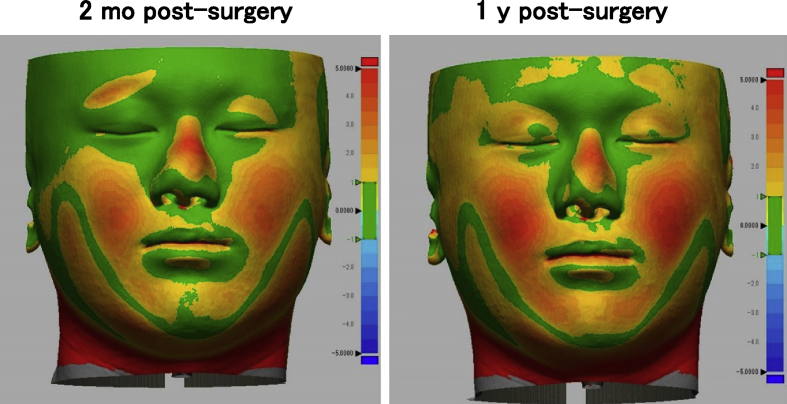
One woman and two men who presented with cleft lip–nose deformity were enrolled between December 13, 2010 and February 6, 2012. A twenty-five year old female (patient #1) was suffering from a right cleft lip (Fig. 2). The second patient (#2) was the twenty-one year old male, who was suffering from a bilateral cleft lip and palate. In this patient, a lip switch flap was also performed, in addition to the transplantation of tissue-engineered cartilage (Fig. 3). Patient #3 was a 22-year old male suffering from a left cleft lip and palate (Fig. 4). In this patient, we observed calcification in the area of the tissue-engineered cartilage (Fig. 4 bottom).
Fig. 2.
Facial changes of patient #1. The shape of nose was improved (top), while the changes of 3D CT images were not detected (bottom).
Fig. 3.
Facial changes of patient #2. The transplantation of tissue-engineered cartilage and a lip with flap resulted in an improved nose shape (top). Prominent changes were not observed in 3D CT images (bottom).
Fig. 4.
Facial changes of patient #3. The nasal shape was improved (top), although some calcification was noted in the areas of tissue-engineered cartilage, postsurgery (bottom, arrow).
2.2. Outcomes
The postsurgical courses were followed up with facial photographs, CT and MRI images. The images were taken before the transplantation of the tissue-engineered cartilage, as well as 2 months and 1 year post-transplantation.
All of the CT images were generated by a helical CT scanner (Aquilion®, Toshiba, Tokyo, Japan) with unified parameters; reconstruction interval of 1 mm; tube current of 300 mA at 120 kV. CT data were set to 150 in bones, and −150 in skin. STL data of skins and bones were reconstructed by volume rendering of the CT data (OsiriX, OsiriX Imaging Software). The STL data in both pre-surgery and post-surgery were superimposed, under the adjustment on frontonasal suture and bilateral zygomatic bases. The surface distance was calculated, which was presented by color mapping (Geomagic Design X, 3Dsystems). In all patients, MRI was performed on a 3.0-T MRI system (Discovery MR750w, GE Healthcare, Milwaukee, WI, USA) with a 12-channel head coil. The following MR images were acquired: T1-weighted images (FOV 16–22 cm, TR/TE 380–820/10–15 ms); T2-weighted images (FOV 16–22 cm, TR/TE 3500–4212.40/88–106.56 ms); fat suppressive T2-weighted images (FOV 22 cm, TR/TE 3500–5060/103.49–109.01 ms).
This study was registered with the UMIN-CTR, number UMIN000005472.
3. Results
We did color mapping of the surface distances between pre-surgery and post-surgery in 3D shape model measured from CT images. The bridge of nose was colored orange to red (>2 mm), in all patients (Fig. 5, Fig. 6, Fig. 7). The height of nose was increased post-surgery, in whole length of nasal dorsum, which was localized in the medial line of the nasal dorsum. The affected curves of nasal dorsum were not detected after the transplantation. This augmentation of nose was maintained by 1 year post-surgery. Patients #1 and #2 showed significant improvement in nasal tip shapes, and well-balanced nasal alae (Fig. 5, Fig. 6).
Fig. 5.
Color mapping of surface distance between pre- and post-surgery in patient #1.
Fig. 6.
Color mapping of surface distance between pre- and post-surgery in patient #2.
Fig. 7.
Color mapping of surface distance between pre- and post-surgery in patient #3.
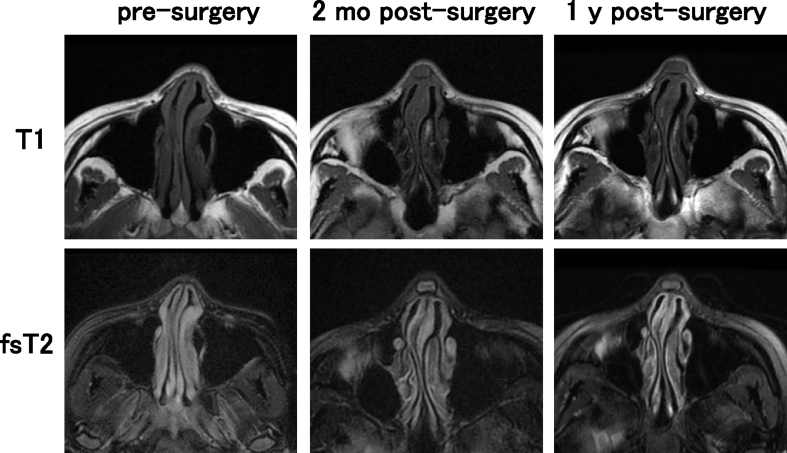
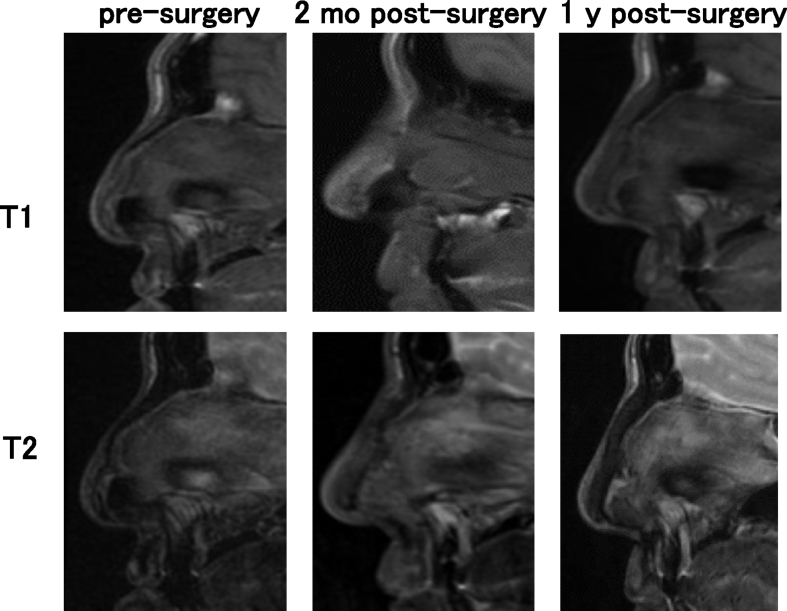
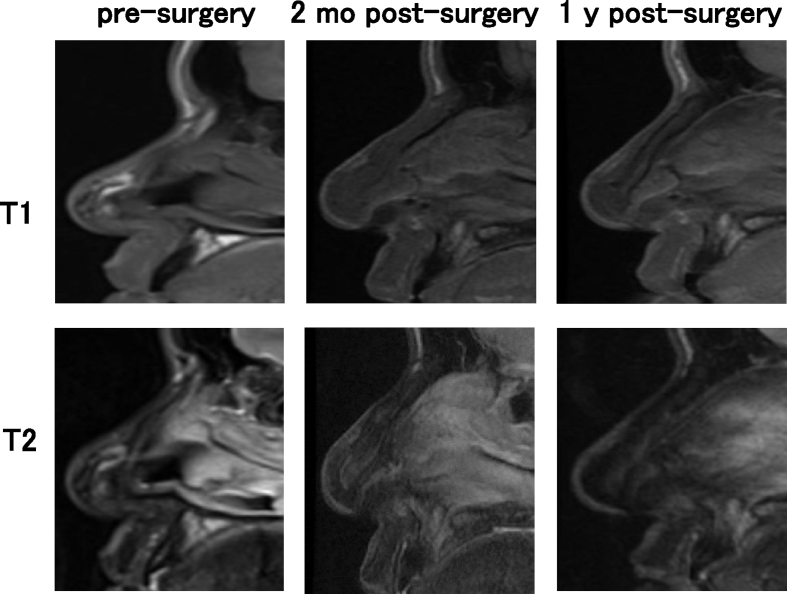
In the findings of MRI, the images of transplanted cartilage had been observed until 1 year post-surgery. In the axial view, the areas of iso-intensity was observed in the subcutaneous areas of nasal dorsum in T1 images, while those of iso- to high intensity was detected in the same areas of fsT2 images (Fig. 8). The sagittal view displayed the obscure images of the tissue-engineered cartilage. T1 images showed longitudinal shapes of the transplanted cartilage with iso-intensity. In the T2 images, the constructs showed iso- to high intensity 2 months post-surgery. In Fig. 9, Fig. 10, the T2 images of 2 months post-surgery showed rather high intensity in the areas of transplants. However, the intensity in the same areas of the T2 images tended to decrease, 1 year post-surgery. The images of transplants were seemingly not straight when viewed from the longitudinal plain, and may have shown gentle adaptation to the surrounding nasal bones and alar cartilage tissues (Fig. 9, Fig. 10, T1, 1 y post-surgery). No significant changes in intensity due to calcification were noted when comparing Fig. 9, Fig. 10.
Fig. 8.
MRI images of patient #1. T1 and fat suppressive T2 (fsT2) images in axial view.
Fig. 9.
MRI images of patient #2. T1 and T2 images in sagittal view.
Fig. 10.
MRI images of patient #3. T1 and T2 images in sagittal view.
4. Discussion
Cleft lip and palate-associated nose deformity entails curvature of the nasal dorsum, hypoplasia and deviation of columella, and dropped nasal alae. Those complicated deformities make treatment increasingly difficult [6]. We engineered the rod-shaped, implant-type tissue-engineered cartilage and transplanted it into the nasal dorsum of the patients [9].
A variety of imaging techniques have been implemented to analyze nose shapes, including oriented facial photographs and cephalograms [9]. Both were projected as 2D images and thus disadvantageous for full evaluation. Moire fringes [14] and laser measurement [15] were potential candidates for 3D evaluation, however, the requirement for specific equipment prevented it from being utilized to perform standard measurements. Thus, we used CT images. The CT images of the surface shape were extracted, 3-dimensionally reconstructed, and superimposed to calculate the 3D surface changes. The superposition data become inaccurate if the baseline of superimposing for comparison is set at an unstable landmark. Considering that deviation of baseline can be minimized by utilizing the facial bones excluding the mandible, we selected the following 2 points: the frontonasal suture and bilateral zygomatic bases, which resulted in successful measurement of changes without a severe deviation of the skin other than the noses (Fig. 5, Fig. 6, Fig. 7). In all 3 patients, an approximate 2 mm augmentation extending over nearly the whole length of the nasal dorsum was observed in the median line without displaying any excessive curvature or deviation of the augmented region. Those findings suggested that the tissue-engineered cartilage was appropriately inserted and that no resorption or abnormal enlargement occurred in the body. Although warping appears less than 3 months [16], no obvious lateral deformity of the nasal dorsum was noticed at one year post-surgery in the present study. Warping of the transplanted costal cartilage is regarded to reflect the original shape and its anisotropy. In contrast, the tissue-engineered cartilage had the ability to retain a sustainable morphology due to it being created by homogenously dispersing chondrocytes isolated from cartilage tissue in a rigid scaffold material.
No significant resorption was noted in the nose tip of the present study. In several of the patients using autologous iliac bones, skin tension progressed the resorption, gradually leading to a turned-up nose [17]. The slow metabolic turnover, characteristic of cartilage tissues, defines cartilage resorption as an unlikely event to occur. Consistent with this fact, no obvious resorption of tissue-engineered cartilage was noted at the nose tip, based on the 3D measurement of CT images (Fig. 5, Fig. 6, Fig. 7), suggesting its contribution to improvement of the nose tip morphology. In order to refrain from excessive radiation exposure, we took CT images only by one year post-surgery, in this study. Otherwise, the cephalograms were taken till three years in the present series, which showed more than 2 mm of nose augmentation maintained for 3 years post-surgery, as measured in the 2D method [9]. In the future project, we will prove that the effective augmentation of nose and the improvement in 3D nose shapes continue for a longer period, by CT data.
The sagittal cross-sectional images from MRI scans with iso-intensity in T1 and iso- to high intensity in T2, revealed the morphological shape of the transplanted cartilage in its entirety (Fig. 9, Fig. 10). The findings resembled those of experimental transplantation in nude rats [18]. However, it appeared different from the morphology of repaired actual articular cartilage reported to show prominently high intensity on fsT2 images of MRI [10]. These discrepancies may have resulted from differences in the physical properties or composition of the scaffold material. Due to the latent effects of biodegradation of scaffold material over the course of several years, it may be necessary to continue course observation. Thus, the MR images, especially in a sagittal view, were regarded as a useful tool to grasp the in vivo image of the transplant that was shaped rather longer in a longitudinal axis. The further and fine follow-ups by MRI may enable to visualize the time-course changes in whole shapes and biochemical properties of the tissue-engineered cartilage transplanted into a body.
5. Conclusions
In the present study, the tissue-engineered cartilage was subcutaneously inserted, and the 3D surface nasal shape was evaluated using CT images one year after surgery to confirm the maintenance of the improved morphology in the dorsum and apex of the nose. Increased sample size of patients will be necessary in accruing additional MRI data on the transplanted tissue-engineered cartilage. Further investigation of the correlation between the imaging and pathological data is necessary, which will be accommodated by the in vivo evaluation method. A multiple center clinical trial on this implant-type regenerated cartilage is now being performed. We are planning its industrialization after approval by the government.
Conflict of interest
Atsuhiko Hikita MD, PhD. and Yukiyo Asawa DVM, PhD are members of the endowed chair in The University of Tokyo supported by Fujisoft incorporated. Other authors have nothing to disclose.
Acknowledgments
We would like to thank Dr. Hirokazu Tsuno, Mr. Yutaka Nagura and Dr. Ryo Orii for their valuable support in blood sampling and general anesthesia, and Mr. Motohiro Harai for his useful discussion about quality control and the delivery system. We also like to thank Ms. Mikako Harata and Ms. Michiko Ishihara for their English correction and technical assistance. This study was supported by Grants-in-Aid for Medical Research and Development Programs Focused on Technology Transfer (AMED A-STEP, D07-05), and Research Project for Practical Applications of Regenerative Medicine (AMED).
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Marlovits S., Zeller P., Singer P., Resinger C., Vecsei V. Cartilage repair: generations of autologous chondrocyte transplantation. Eur J Radiol. 2006;57:24–31. doi: 10.1016/j.ejrad.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 2.Ochi M., Uchio Y., Kawasaki K., Wakitani S., Iwasa J. Transplantation of cartilage-like tissue made by tissue engineering in the treatment of cartilage defects of the knee. J Bone Jt Surg Br. 2002;84:571–578. doi: 10.1302/0301-620x.84b4.11947. [DOI] [PubMed] [Google Scholar]
- 3.Wakitani S., Imoto K., Yamamoto T., Saito M., Murata N., Yoneda M. Human autologous culture expanded bone marrow mesenchymal cell transplantation for repair of cartilage defects in osteoarthritic knees. Osteoarthr Cartil. 2002;10:199–206. doi: 10.1053/joca.2001.0504. [DOI] [PubMed] [Google Scholar]
- 4.Sekiya I., Muneta T., Horie M., Koga H. Arthroscopic transplantation of synovial stem cells improves clinical outcomes in knees with cartilage defects. Clin Orthop Relat Res. 2015;473:2316–2326. doi: 10.1007/s11999-015-4324-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pers Y.M., Rackwitz L., Ferreira R., Pullig O., Delfour C., Barry F. Adipose mesenchymal stromal cell-based therapy for severe osteoarthritis of the knee: a phase I dose-escalation trial. Stem Cells Transl Med. 2016;5:847–856. doi: 10.5966/sctm.2015-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pawar S.S., Wang T.D. Secondary cleft rhinoplasty. JAMA Facial Plast Surg. 2014;16:58–63. doi: 10.1001/jamafacial.2013.1562. [DOI] [PubMed] [Google Scholar]
- 7.Ortiz Monasterio F., Ruas E.J. Cleft lip rhinoplasty: the role of bone and cartilage grafts. Clin Plast Surg. 1989;16:177–186. [PubMed] [Google Scholar]
- 8.Tanaka Y., Yamaoka H., Nishizawa S., Nagata S., Ogasawara T., Asawa Y. The optimization of porous polymeric scaffolds for chondrocyte/atelocollagen based tissue-engineered cartilage. Biomaterials. 2010;31:4506–4516. doi: 10.1016/j.biomaterials.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 9.Hoshi K., Fujihara Y., Saijo H., Asawa Y., Nishizawa S., Kanazawa S. Implant-type tissue-engineered cartilage for secondary correction of cleft lip-nose patients: an exploratory first-in-human trial. J Clin Trials. 2017 doi: 10.1016/j.reth.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Recht M.P., Goodwin D.W., Winalski C.S., White L.M. MRI of articular cartilage: revisiting current status and future directions. AJR – Am J Roentgenol. 2005;185:899–914. doi: 10.2214/AJR.05.0099. [DOI] [PubMed] [Google Scholar]
- 11.Takahashi T., Ogasawara T., Kishimoto J., Liu G., Asato H., Nakatsuka T. Synergistic effects of FGF-2 with insulin or IGF-I on the proliferation of human auricular chondrocytes. Cell Transpl. 2005;14:683–693. doi: 10.3727/000000005783982675. [DOI] [PubMed] [Google Scholar]
- 12.Yamaoka H., Tanaka Y., Nishizawa S., Asawa Y., Takato T., Hoshi K. The application of atelocollagen gel in combination with porous scaffolds for cartilage tissue engineering and its suitable conditions. J Biomed Mater Res A. 2010;93:123–132. doi: 10.1002/jbm.a.32509. [DOI] [PubMed] [Google Scholar]
- 13.Takato T., Yonehara Y., Mori Y., Susami T. Use of cantilever iliac bone grafts for reconstruction of cleft lip-associated nasal deformities. J Oral Maxillofac Surg. 1995;53:757–762. doi: 10.1016/0278-2391(95)90325-9. [DOI] [PubMed] [Google Scholar]
- 14.Kawai T., Natsume N., Shibata H., Yamamoto T. Three-dimensional analysis of facial morphology using moire stripes. Part II. Analysis of normal adults. Int J Oral Maxillofac Surg. 1990;19:359–362. doi: 10.1016/s0901-5027(05)80082-7. [DOI] [PubMed] [Google Scholar]
- 15.Verze L., Bianchi F.A., Ramieri G. Three-dimensional laser scanner evaluation of facial soft tissue changes after LeFort I advancement and rhinoplasty surgery: patients with cleft lip and palate vs patients with nonclefted maxillary retrognathic dysplasia (control group) Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;117:416–423. doi: 10.1016/j.oooo.2013.12.406. [DOI] [PubMed] [Google Scholar]
- 16.Wilson G.C., Dias L., Faris C. A comparison of costal cartilage warping using oblique split vs concentric carving methods. JAMA Facial Plast Surg. 2017 doi: 10.1001/jamafacial.2017.0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sugiyama M., Nakatsuka T., Saijo H., Fujihara Y., Kanno Y., Hikita A. Clinical findings of a cantilever iliac bone graft for secondary correction of cleft lip-nose deformities. J Craniofac Surg. 2017 doi: 10.1097/SCS.0000000000004070. [in press] [DOI] [PubMed] [Google Scholar]
- 18.Fujihara Y., Nitta N., Misawa M., Hyodo K., Shirasaki Y., Hayashi K. T2 and apparent diffusion coefficient of MRI reflect maturation of tissue-engineered auricular cartilage subcutaneously transplanted in rats. Tissue Eng C Methods. 2016;22:429–438. doi: 10.1089/ten.TEC.2015.0291. [DOI] [PubMed] [Google Scholar]