Abstract
Anisakis simplex spp. sensitization rates have increased worldwide, with a significant impact on health-care systems. To date, no clear-cut diagnostic criteria and laboratory algorithm have been established, so anisakiasis still represents an under-reported health problem whose clinical manifestations, when present, mimic the much more common allergic and digestive disorders. Aim of the study was to systematically review the available literature on the prevalence of sensitization against Anisakis in the general population and in specific population groups, taking into account the impact of the different available diagnostic techniques on the epidemiological data. Following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement, relevant papers reporting Anisakis sensitization epidemiological data were found covering a period ranging from 1996 to February 2017. Overall, 41 studies comprising 31,701 participants from eleven countries were included in the qualitative synthesis. General asymptomatic population resulted sensitized to Anisakis in 0.4 to 27.4% of cases detected by means of indirect ELISA or ImmunoCAP specific IgE detection, and between 6.6% and 19.6% of the samples by Skin prick test (SPT). Occupationally exposed workers (fishermen, fishmongers and workers of fish-processing industries) documented specific IgE between 11.7% and 50% of cases, whereas SPT positivity ranged between 8% and 46.4%. Symptomatic allergic patients to any kind of allergen were found to be positive to Anisakis specific IgE detection between 0.0% (in children with mastocytosis) to 81.3% (among adults with shellfish allergy). Results highlighted that hypersensitivity prevalence estimates varied widely according to geographical area, characteristics of the population studied, diagnostic criteria and laboratory assays. Further studies are needed to overcome the documented misdiagnosis by improving the diagnostic approach and, consequently, providing more affordable estimates in order to address public health interventions on populations at high risk of exposure to Anisakis and to tailor health services related to specific groups.
Introduction
During the last decades, progress in the food industry and globalization have markedly increased the exposure to new allergenic sources that not always are adequately pointed out [1]. Coupled with changes in eating habits, including widespread consumption of raw, marinated or smoked fish, a quota of food allergies of unknown origin in the general population may be due to sensitization to Anisakis spp., representing a public health issue of growing importance [2,3]. Moreover, occupational contact was associated with Anisakis sensitization and allergic symptoms among fish-processing workers and fishmongers [4,5].
Humans can become accidental non-permissive hosts of the Anisakis parasite by eating parasitized raw or undercooked fish containing larvae in stage 3 [6,7]. Within hours after being ingested, Anisakis larvae penetrate the mucosal layers of the gastrointestinal tract, causing direct tissue damage that may lead to the zoonotic disease known as anisakiasis. This acute gastrointestinal form of Anisakis infection is usually transient, with the worm dying within a few weeks. It is manifested by clinical symptoms ranging from nausea, vomiting, diarrhoea, mild to severe abdominal pain and intestinal obstruction [8], mimicking other much more common gastrointestinal disturbances, such as acute appendicitis, gastric ulcer, or tumours, thus making diagnosis of anisakiasis extremely difficult.
Moreover, Anisakis is implicated in allergic IgE-mediated reactions, occurring after secondary exposure to the parasite, such as urticaria, angioedema, asthma and, rarely, anaphylaxis in highly sensitized people [2, 9–12]. Not by chance, in the past, allergic reactions to Anisakis have been mistaken for other entities such as acute urticaria or fish allergy [13]. Of interest, high levels of specific IgE for Anisakis allergens were also detected in healthy individuals without any clinical symptom.
The current diagnostic algorithm of Anisakis-related allergy has been based upon suggestive anamnesis (appearance of symptoms few hours after raw fish intake) along with positive skin prick testing, enzyme-linked immunosorbant assay (ELISA), ImmunoCAP or immunoblotting determination of antigen-specific IgE and exclusion of fish allergy, but the high number of false positives due to cross-reactivities with numerous panallergens has underlined the need to improve the diagnostic approach [14–17].
Often, these misdiagnosis lead to a domino cascade of useless diagnostic tests with significant healthcare costs [18].
The significant impact of Anisakis sensitization in the general population and in specific occupational settings (mainly allergic patients and fishing industry workers) has been stressed by several studies, particularly the ones with the largest sample size, held in Japan, Spain and Italy, documenting how Anisakis was a leading cause of food allergies more frequently than seafood itself [4,19–21]. Furthermore, sensitization to Anisakis was correlated not only with ingestion of contaminated fish, but also among workers whose occupation consisted of frequent handling of raw fish or fishmeal [4], also including cooks and restaurant workers [22–25].
The accurate assessment of Anisakis hypersensitivity prevalence plays a pivotal role to tailor health services and public initiatives according to the needs of the population, particularly in order to plan disease surveillance, ensure sufficient resources to cope with the burden of disease and evaluate trends over time [6]. Also, differences in diagnostic techniques and characteristics of populations enrolled led to conflicting reports among various geographical areas [8].
We performed a systematic review of the available literature on Anisakis sensitization prevalence in general population and other population strata, including occupationally exposed workers, taking into account the impact of the different available diagnostic techniques on the epidemiological data.
Material and methods
Search strategy
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)
Guidelines [26] were followed to conduct the systematic review of the literature (S1 Table). Ethics board review was not sought because this review used only publically available information.
Database search
A systematic review of peer-reviewed English-language literature for Anisakis spp. sensitization prevalence data was conducted through a search of Medline and Scopus databases. Initially, free text words representing broad concept of “Anisakis allergy” were used to identify the keywords (for example, Medical Subject Headings, MeSH) for subject searching. Then, a combination of MeSH terms and free text words were arranged in the following research string with OR and AND logical operators: Anisakis AND (prevalence OR epidemiology) AND (allergy OR hypersensitivity OR immunization OR sensitivity OR sensitization OR ELISA OR skin prick test OR ImmunoCAP OR Immunoblot OR diagnostic techniques). Reference lists of the articles included in the analysis and of others relevant to the topic were hand-searched to identify additional potentially relevant publications, until no new information was found.
Other sources
Grey literature was identified by searching for conference or meeting abstracts and proceedings. The literature was last searched on February the 6th 2017.
Inclusion and exclusion criteria
All articles meeting the following criteria were screened and then assessed for eligibility: peer reviewed manuscripts, published from 1996 to February 2017, reporting Anisakis sensitization prevalence estimates, a description of the population involved, the techniques used to test for immunization and the number of people tested.
Reports of analytical studies (cross-sectional studies, prospective or retrospective) were included, with no restriction on age or type of population. Review articles, conference abstracts, editorials and case reports were excluded.
Screening
After removal of duplicates, the records were screened by two reviewers (CM and DDR) in three levels. The first level included title screening, the second level included abstract screening and the third level included full text screening. For each level, the reviewers separately screened the records. Any disagreement was resolved by consensus with a third author (WM). After screening, studies were assessed for eligibility and final selection.
Study quality assessment process
Quality assessment of thirty-seven studies included was performed by using an adapted version of the Joanna Briggs Institute Prevalence Critical Appraisal Tool [27], which was tailored to the objective and primary outcome measures of this review by modifying in order to account for specific Anisakis sensitization test criteria. Each study was assessed for ten criteria (Table 1): sample representativeness; participants recruitment; sample size; description of participants and setting; response rate; objective, reliable measurement of Anisakis sensitization; reliability of diagnostic techniques; appropriateness of statistical analysis, confounding factors/subgroups/differences identified and accounted for; identification of subpopulations using objective criteria (S2 Table). Being the maximum score obtainable equal to 14, a score of 7 was considered as cut off between middle-low and middle-high study quality.
Table 1. Criteria for the quality assessment of the studies (adapted from Joanna Briggs Institute Prevalence Critical Appraisal Tool).
| Criteria | Score (Maximum score = 14) |
|---|---|
| 1. Sample representativeness | Adequate = 1, Not Adequate = 0, NA |
| 2. Participants recruitment | Random = 1, all other methods = 0, NA |
| 3. Sample size | ≥200 = 1, <200 = 0, NA |
| 4. Description of participants and setting | Adequate = 1, Not Adequate = 0, NA |
| 5. Response rate, % | <50% = 0, 50–80% = 1, >80% = 2 |
|
6. Objective, reliable measurement of Anisakis sensitization |
3 diagnostic criteria (anamnestic, clinical, laboratoristic) = 3; only 2 up to 3 criteria = 2; only one criteria = 1 |
| 7. Reliability of diagnostic techniques | Antigens used specified in the text = 2; only anamnesis = 0, all the other measurement = 1 |
| 8. Appropriateness of statistical analysis | Adequate = 1, Not Adequate = 0, NA |
|
9. Confounding factors/subgroups/differences identified and accounted for |
Adequate = 1, Not Adequate = 0, NA |
|
10. Identification of subpopulations using objective criteria |
Adequate = 1, Not Adequate = 0, NA |
When only the abstract was available, quality assessment could not be performed.
Data extraction
Data were extracted using a data extraction MS Excel sheet. Data extraction included authors, year of publication, year of study, study location, study design, statistical measures, study settings, samples size, the characteristics of the studies’ participants, as age and females/males ratio, as well as diagnostic techniques and criteria employed to define Anisakis sensitization and allergy and relative prevalence estimates. DDR conducted data extraction, while CM performed the analysis of the studies’ quality.
Study protocol
The study protocol has been registered with PROSPERO, number CRD42017057316.
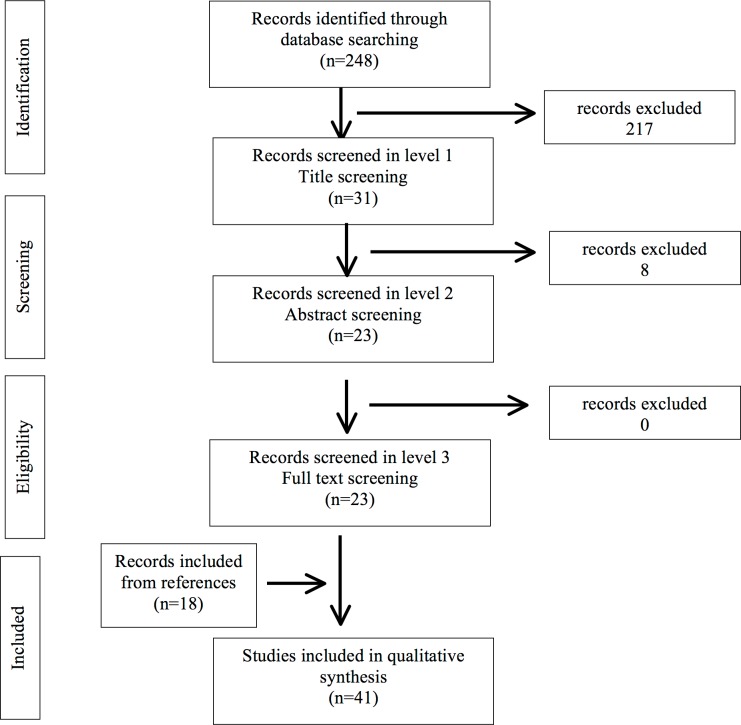
A total of 248 records were identified searching in Medline and Scopus databases.
After title screening, 217 records were excluded. Of the remaining 31 manuscripts, 8 were removed subsequently to abstract evaluation. In the latest phase, full text assessment led to inclusion of all the remaining 23 manuscripts, and 18 more articles fulfilling the inclusion criteria found in the reference lists were added (Fig 1) (S1 Database).
Fig 1. Flow diagram for selection of studies on Anisakis sensitization prevalence.
Diagnostic techniques employed
Skin prick test (SPT)
Sensitization to Anisakis is ruled out by the appearance of a >3 mm diameter wheal on the volar aspect of each subject's forearm after scratching the skin in the presence of a dilution of Anisakis extract (obtained by the centrifugation of total larvae in phosphate-buffered saline for 15 minutes at 1500 g) [28, 29].
ImmunoCAP, UniCAP-100, Radio-Allergo-Sorbent Test (RAST)
Total immunoglobulins (Igs), IgE, IgM, IgA1, IgG1, IgG4, antibodies against Anisakis crude extract, excretory-secretory antigens and recombinant antigens Ani s1, Ani s3, Ani s5, Ani s9, and Ani s10 are first detected by incubation with subjects’ serum samples and then revealed using an anti-human Igs labelled with radioactive or fluorescent marker [11, 30–33].
Indirect ELISA
Specific anti-Ani s1 and Ani s7 IgE antibodies are detected in patients’ sera after adding diluted Anisakis antigens to ELISA plates, and then incubating with secondary antibodies coupled to an enzyme. After washing, so that excess of unbound antibodies can be removed, a substrate is added, and remaining enzymes elicit a chromogenic or fluorescent signal, which is proportional to the antibody-antigen complexes and can be measured as optical density (OD) [34].
Antigen capture ELISA
It is a variation of indirect ELISA in which the serum to be tested is added to wells containing O-deglycosylated A. simplex antigen bound by the immobilized monoclonal antibody UA3, in order to detect specific IgG1 and IgE [35].
rAni s1, rAni s7 ELISA
Specific anti-Anisakis IgE antibodies are detected by indirect ELISA, with rAni s 7 or rAni s 1 as the target. After incubation of the plates and blocking of nonreactive sites, undiluted serum is added to each well and the specific IgE detected [36].
Immunoblotting
Anisakis specific IgE detection is performed by means of sodium dodecylsulfate-polyacrylamide gel electrophoresis with a dilution of Anisakis extract or recombinant Ani s1, Ani s3, Ani s5, Ani s9 and Ani s10. Proteins are afterwards transferred to nitrocellulose membranes and incubated overnight with diluted sera from patients in incubation buffer. After washing, the membranes are incubated with appropriate dilution of monoclonal labelled antihuman immunoglobulins [19, 37].
Basophil activation test (BAT)
Flow-cytometry expression of CD63 on activated basophils is measured on whole blood sample after incubation in a water bath with Anisakis crude extract. Samples are lysed, washed and re-suspended to be measured in a flow cytometer after staining cells with 20 μL of CD63-FITC ⁄ CD123-PE ⁄ anti-HLA-DR-PerCP reagent mixture cocktail. Activated basophils were finally identified using anti-CD123, anti-HLA-DR and anti-CD63 monoclonal antibodies [38].
Results
The characteristics of the 41 studies reviewed in the qualitative analysis are summarized in Table 2.
Table 2. Characteristics of the n. 41 studies included in the systematic review.
| Author, year | Country | Study design | Study period | Study setting | Study participants | N° of participants | Age mean (SD) or median (IQR), range |
Female n/N (%) |
|---|---|---|---|---|---|---|---|---|
| Abattouy, 2013 | Morocco | Cross sectional | Not provided | Public clinical analysis laboratories and general practitioner | Inhabitants and fishing industry workers from 2 coastal cities | 333 | 38.4 22–70 |
156/333 (46.8) |
| Anadon, 2010 | Spain | Cross sectional | 1995–2001 | Allergy service of Madrid hospital | Serum samples of Madrid allergic inhabitants |
495 | 44.3 5–81 |
322/495) (65) |
| Andreu-Ballester, 2008 | Spain | Case-control | Not provided | 1 hospital | Appendectomized patients and non appendectomized patients who presented to the emergency department | 160 (80 appendectomized patients and 80 non appendectomized patients) |
39.2 (14) 20–75 |
76/180 (47.5) |
| Añíbarro, 2007 | Spain | Cross-sectional | Not provided | 1 allergy unit covering 400.000 inhabitants public health area |
Food allergic patients | 436 | 46 (13.8) (mean age onset of symptoms) |
Not provided |
| Asero, 2009 | Italy | Cross-sectional | 2007 | 19 allergy outpatient clinics | Food allergic patients | 1,110 | 31 12–79 |
719/1,110 (64.7) |
| Bernardini, 2000 | Italy | Cross-sectional | Not provided | 1 allergy unit | Children with allergic symptoms | 805 | 7.9 (3.8) 0.52±17.61 |
324/805 (40.2) |
|
Caballero, 2012 |
Spain | Cross-sectional | Not provided | 1 allergy unit | Tolerant patients with suspected not-fish-related allergy and patients with allergy to A. simplex. |
99 (tolerant patients with suspected not-fish-related allergy) 35 (patients with allergy to A. simplex) |
36.2 (tolerant patients with suspected not-fish-related allergy) 52.5 (patients with allergy to A. simplex). |
Not provided |
|
Consortium AAITO-IFIACI Anisakis, 2010 |
Italy | Cross-sectional | 2010 | 34 allergy units | Suspected allergy patients | 10,570 | Not provided | Not provided |
| Daschner, 2005 | Spain | Cross-sectional | Not provided | 1 allergy unit | Chronic urticaria patients | 135 | 41.5 (15.4) | 91/135 (67.4) |
| del Pozo, 1997 | Spain | Cross-sectional | Not provided | 1 allergy unit | Urticaria/angioedema patients | 100 | 37.4 18–75 |
63/100 (63.0) |
| Del Rey Moreno, 2006 | Spain | Cross-sectional | 2000 | 1 hospital laboratory | Random healthy blood donors | 77 | Not provided | Not provided |
| Estrada Rodriguez, 1997 | Spain | Cross-sectional | Not provided | 1 hospital laboratory | Not provided | 66 | Not provided | Not provided |
| Falcao, 2008 | Portugal | Case-control | Not provided | Immuno- allergology and surgery units of the largest paediatric hospital in Porto |
Cases with of acute urticaria Controls among programmed surgery patients |
200 (cases with of acute urticarial). 200 (controls among programmed surgery patients). 400 overall |
6–18 | 66/200 (33.0) for cases. 92/200 (46.0) for controls. Overall 158/400 (39.5) |
| Figueiredo, 2013 | Brazil | Cross-sectional | 2010 | 1 military facility | Healthy adult affiliated with a military facility | 67 | 40 ± 8.4 years (median) | Not provided |
| Figueiredo, 2015 | Brazil | Cross-sectional | 2009–2010 | 2 perinatal centers (1 high-risk birth unit and 1low-risk birth unit) |
Mother-newborn pairs | 139 from LRBU 170 from HRBU 309 overall |
24.80 (LRBU) 26.19 (HRBU) overall range 15–42 |
309/309 (100) |
| Frezzolini, 2010 | Italy | Case-control | Not provided | Laboratory of immunology and allergology unit | Chronic urticaria patients, atopic patients, healthy controls | 57 chronic urticaria patients, 22 atopic patients, 20 healthy controls |
Chronic urticaria patients: 42 (11) 24–54 Not provided for controls |
Chronic urticaria patients: 49/57 (86.0) Not provided for controls |
| Garcia, 1997 | Spain | Case-control | Not provided | 1 hospital | Cases: patients with urticaria, angioedema, or anaphylaxis; controls: healthy blood donors |
61 cases, 51 controls |
47 21–72 for cases, 41 18–65 for controls |
39/61 (63.9) for cases, 22/47 (46.8) for controls |
| García-Palacios, 1996 | Spain | Cross-Sectional | Not provided | 1 hospital laboratory | Randomly selected adults showing no clinical suspicion of anisakidosis | 1,008 | Not provided | Not provided |
| Garcia-Perez, 2015 | Spain | Case-control | 2010–2013 | 1 hospital | gastric cancer patients, healthy controls | 47 cases, 47 controls | 70 (48–92) for cases, 65 (46–83) for controls |
24/47 (51.1) for cases, 21/47 (44.7) for controls |
| Gomez, 1998 | Spain | Case-control | 1989–1996 | 1 allergy unit | Cases with eosiniphilic gastroenteritis, controls without digestive disorder, controls with digestive disorder different from eosinophilic gastroenteritis |
10 cases, 149 controls without digestive disorder, 10 controls with digestive disorder different from eosinophilic gastroenteritis |
50 for cases, not provided for controls | 4/10 (40.0) for cases, not provided for controls |
| González de Olano, 2007 | Spain | Cross-sectional | 2003–2005 | 1 Allergy unit | adults with mastocytosis, children with mastocytosis, controls |
163 adults with mastocytosis, 47 children with mastocytosis, 50 controls | 43 (median) (18–75) for adults with mastocytosis8 (median) (0.6–14) for children with mastocytosis, Range 2–70 for controls |
88/163 (54.0) for adults with mastocytosis 17/47 (36.2) for children with mastocytosis, not provided for controls |
| Gonzalez Munoz, 2005 | Spain | Cross-sectional | Not provided |
1 Department of Immunology | consecutive patients divided into Anisakis sensitized (symptoms + IgE+), chronic urticaria/abdominal pain unrelated to fish ingestion |
88 overall (37 Anisakis sensitized, 51 with chronic urticaria/abdominal pain unrelated to fish ingestion) | median 34 (IQR = 28–48) |
60/88 (68.2) |
| Guillén-Bueno, 1999 | Spain | Cross-sectional | Not provided |
1 hospital (gastroenterology Service) | Crohn’s disease patients, random controls | 73 cases, 251 controls | 35.1 (12.2) 15–73 |
42/73 (57.5) |
| Gutierrez, 2002 | Spain | Cross-sectional | 1996–1997 | 1 hospital (gastroenterology Service) | Gastrointestinal diseases patients, patients with digestive haemorrhaging, patients with Crohn’s disease, patients with digestive cancer, patients with appendicitis. |
57 gastrointestinal diseases patients, 19 patients with digestive haemorrhaging, 30 patients with Crohn’s disease, 4 patients with digestive cancer 5 patients with appendicitis. |
42.4 (17.6) gastrointestinal diseases patients, 54.3 (15.9) patients with digestive haemorrhaging, 37.2 (11.5) patients with Crohn’s disease, 71.3 (5.7) patients with digestive cancer, 24.8 (4.7) patients with appendicitis. |
Not provided |
| Heffler, 2016 | Italy | Cross-sectional | 2010–2012 | 1 Allergy unit | Consecutive allergic patients | 3,419 | 34.3 3–88 |
2,114/3,419 (61.8) |
| Kim, 2011 | South Korea | Cross-sectional | Not provided |
3 hospitals laboratories | Non allergic patients admitted for health examinations | 498 | from teens to 98 | 269/498 (54.0) |
| Kimura, 1999 | Japan | Cross-sectional | 1994–1997 | Various laboratories throughout Japan | Allergic patients | 2,108 | Not provided |
Not provided |
| Lin, 2012 | Norway | Cross-sectional | Not provided |
1 university hospital, allergy laboratory |
blood donors, suspected allergy patients |
100 blood donors, 798 suspected allergy patients |
Not provided |
Not provided |
| Lin, 2014 | Cross-sectional | Not provided |
1 university hospital, allergy laboratory |
Blood donors, patient with total IgE levels ≥1000 kU/L |
993 blood donors, 414 patient with total IgE levels ≥1000 kU/l |
Not provided |
Not provided |
|
|
Mazzucco, 2012 |
Italy | Cross-sectional | 2009 | 1 hospital laboratory | Fishing industry workers | 94 | 42.1 (12) | 16/94 (17.0) |
| Mladineo, 2014 | Croatia | Cross-sectional | 2010–2011 | 1 county secondary healthcare provider Medicine-biochemical Laboratory |
Unpaid randomly selected volunteer healthy subjects | 500 | 58.1 | 242 (48.4) |
| Montoro, 1997 | Spain | Cross-sectional | 1995 | 1 hospital immunology and allergy service | Acute recidivous urticaria patients who usually eat fish or other seafood | 25 | 39.3 (19.8) 11–77 |
16/25 (64.0) |
| Nieuwenhuizen,2006 | South Africa | Cross-sectional | Not provided |
1 laboratory | Fishing industry workers | 578 | Not provided |
Not provided |
| Pascual, 1996 | Spain | Cross-sectional | Not provided |
1 laboratory | Patients with increased levels of serum total IgE |
73 | Not provided |
Not provided |
| Puente, 2008 | Spain | Cross-sectional | Not provided |
1 laboratory | Allergic residents of Madrid, non-allergic subjects divided in patients with non-digestive non-allergic pathologies unrelated to anisakiosis and healthy residents of Madrid |
86 allergic residents of Madrid, 314 non-allergic subjects divided in 50 patients with non-digestive non-allergic pathologies unrelated to anisakiosis, and 264 healthy residents of Madrid |
Not provided for allergic residents of Madrid; 57.6 (20–85) for patients with non-digestive non-allergic pathologies unrelated to anisakiosis, 32.9 (18–65) for healthy residents of Madrid |
Not provided |
| Purello-D’Ambrosio, 2000 | Italy | Cross-sectional | Not provided |
1 Laboratory | males in daily contact with fish, non atopic healthy males |
28 males in daily contact with fish, 15 non atopic healthy males | 30.6 (18–48) for males in daily contact with fish, 32.5 (20–44) for non atopic healthy males |
0 (0.0%) |
| Rodriguez, 2000 | Spain | Cross-sectional | Not provided |
1 allergology clinic | Drug allergy patients | 53 | 48.0 (16.7) 17–75 |
36/53 (67.9) |
| Toro, 2004 | Spain | Cross-sectional | 1998 | 1 hospital | Dyspeptic patients | 174 | 49.3 (15.1) 21–80 |
83/173 (48.0) |
| Uga, 1996 | Indonesia | Cross-sectional | 1992–1993 | 1 hospital | Hospital visitors for diarrhea or routine check-ups | 244 | 35 1–80 |
120/244 (49.2) |
| Valinas, 2001 | Spain | Cross-sectional | Not provided |
1 laboratory | Normal unpaid volunteer healthy blood donors | 2,801 | Not provided |
Not provided |
| Ventura, 2013 | Italy | Cross-sectional | Not provided |
1 allergology unit | Adult allergic patients | 919 | 17–83 | 622/919 (67.7) |
Thirty-four studies followed a cross-sectional design, while the remaining ones (n = 7) were designed as case-control study. Twenty-two studies were made on symptomatic allergic population and among them only one specifically enrolled children (Bernardini, 2000) [39]. Three studies included only occupationally exposed population working in the fishing industry (Purello-D’Ambrosio 2000 [40], Nieuwenhuizen N 2006 [5], Mazzucco 2012 [4]), while Abattouy 2013 [41] included both inhabitants and fish workers from two coastal cities in Morocco.
Quality scores ranged from a minimum of 5 (Kimura, 1999) [20] to a maximum of 13 (Anadon 2010 [36], Mladineo, 2014 [42]) up to 14 points scale. Thirty-eight studies were considered of medium-high quality being rated 7 or more, including five studies scoring 10 and three studies scoring 11. Four studies were excluded from quality assessment since only respective abstracts were available (Estrada Rodriguez 1997 [43], Pascual 1996 [44], Rodriguez 2000 [45], Uga 1996 [46]). Extended evaluations on each items analysed for the critical appraisal are in S2 Table.
Data on prevalence, according to different study samples and the diagnostic tests applied, are shown in Table 3.
Table 3. Prevalence of Anisakis sensitization according to different study samples and diagnostic tests.
| Author, year, (reference) | Sample characteristics | Sample size (n) age range (mean, SD) |
Skin Prick Tests % (n), >3 mm threshold |
ELISA/ImmunoCAP % (n), threshold |
Other tests / criteria |
|---|---|---|---|---|---|
| General asymptomatic population (15 studies) | |||||
| Abattouy, 2013 | Random samples | 333 | - | 5.1% | - |
| Del Rey Moreno, 2006 | Healthy blood donors | 77 | - |
22.1% (n = 17) |
Immunoblot 67.5% recognized antigens of A. simplex |
| Figueiredo, 2013 | Healthy military | 67 | - |
20.9% (n = 14) |
- |
| Frezzolini, 2010 | Healthy subjects | 20 | 10.0% | 10.0% | 0.0% CD63 BAT |
| Garcia, 1997 | Healthy blood donors | 51 |
19.6% (n = 10) |
27.4% | Immunoblot 1 75.0% type 4 1.9% (n = 1) type 1 15.0% type 3 |
| García-Palacios, 1996 | Random sera | 1,008 | - |
6.0% (n = 61) |
- |
| Garcia-Perez, 2015 | Healthy controls | 47 | - | 6.4% IgA1, rAni s 1; 10.6% IgA1, rAni s 5 | - |
| Guillén-Bueno, 1999 | Asymptomatic adults | 251 | - | 18.3% | Immunoblot E17, 50-70-250 kDa eosinophilia, leukocytosis |
| Lin, 2012 | Blood donors | 100 | - | 2.0% ImmunoCAP > 0.35 kU/L | - |
| Lin, 2014 | Blood donors | 993 | - |
0.4% ImmunoCAP (0.0% ELISA with rAni s 1 and rAni s 7) |
Immunoblot 40–100 kDa (weaker bands) |
| Mladineo, 2014 | Random healthy | 500 | - |
2.0% indirect ELISA Ani s 1 s 7 |
- |
| Puente, 2008 | Healthy residents | 264 (18–65 years) | - | 11.7% UA3 Ani s 7 | - |
| Purello-D’Ambrosio, 2000 | Healthy donors not occupationally exposed | 15 | 6.6% (n = 1) | 0.0% (n = 0) RAST | - |
| Valinas, 2001 | Healthy blood donors | 2,801 | - | 0.4% UA3 | - |
| Ventura, 2013 | Healthy controls | 187 | 16.0% | - | - |
| Occupationally exposed population, symptomatic and asymptomatic (3 studies) | |||||
| Mazzucco, 2012 | 94 workers in fisheries sector: fishmongers (n = 21), fish industry emplooyees (n = 35), Fishermen/sailors (n = 38) | 94 | - |
11.7% (n = 11) UniCAP-100 |
- |
| Nieuwenhuizen, 2006 | workers employed in 2 large fish-processing workplaces in the Western Cape province of South Africa | 578 |
8.0% (n = 46) |
- | - |
| Purello-D’Ambrosio, 2000 | Fishermen/fishmongers occupationally exposed group | 28 |
46.4% (n = 13) |
RAST 50.0% (n = 14) |
- |
| Symptomatic population with allergies to any kind of allergen (24 studies) | |||||
| Anadon, 2010 | Food allergic; controls non food-related allergic |
493 food allergic; 25 controls non food-related allergic. |
- | CAP-FEIA: 52.7% (n = 195 true positive + 65 false positive) 3 false negative; ELISA rAni s 1 s 7: 40.2% (n = 198) 0 false positive 0 false negative |
- |
| Añíbarro, 2007 | Food allergic | 436 | 12.4% 2 | 12.4% 2 | - |
| Asero, 2009 | Food allergic | 1,110 (12–79 years) | - | - | 0.3% prevalence of systemic reactions/ anaphylaxis |
| Bernardini, 2000 | Suspect allergy | 805 (0.5–17.6 years) | 6.1% (n = 49) | - | - |
| Caballero, 2012 | Sample A: suspect allergy other than fish related; sample B: Anisakis allergic patients (anaphylaxis, angioedema, urticaria or gastrointestinal symptoms few hours after eating undercooked fish) | Sample A: 99; sample B: 35 | Sample A: 18.0%; sample B: 100% | ImmunoCAP: sample A: 17.0%; sample B: 100% | Immunoblot rAni s 1,3,5,9,10: sample A: 15.0%; sample B: 100% |
| Consortium AAITO-IFIACI Anisakis, 2011 |
Suspect allergy | 10,570 | 4.5% (n = 474) | - | Anamnesis + exclusion fish allergy: 0.6% overall; 14.0% of sensitized |
| Daschner, 2005 | Chronic urticaria | 135 | 48.1% (combined SPT+ and IgE+) |
52.6% (only IgE+) 31.8% (only IgG4) |
- |
| Del Pozo, 1997 | Urticaria/angioedema (AE) or anaphylaxis | 100 | 14.0% | 22.0% (>0.7 kU/L) | + symptoms < 6 h after fish ingestion + exclusion other causes: 8.0% real allergy to Anisakis |
| Estrada Rodriguez, 1997 | Asthmatic/urticaria | 66 | - | 19.7% (n = 13) | - |
| Falcao, 2008 | Relapsing acute urticaria | 200 | 16.5% | 6.0% (>0.7 kU/L IgE); 9.0% (>0.35 kU/L IgE) | Combinations SPT IgE: 2.5%
(SPT and >0.7 IgE); 3.0% (SPT and >0.35 IgE); 20.0% (SPT or> 0.7 IgE); 22.5% (SPT or >0.35 IgE) |
| Frezzolini, 2010 | Chronic urticarial, atopic patients |
57 chronic urticarial; 22 atopic patients | 63.0% chronic urticarial; 14.0% atopic patients |
61.0%
(> 0.35 kU/L) chronic urticarial; 18.0% atopic patients |
CD63 BAT 67.0% combined 75% chronic urticarial; 0.0% atopic patients |
| Garcia, 1997 | Subjects with IgE against Anisakis divided into: allergic (anamnesis, the time interval <4 hours between the ingestion of fish and the onset of the reaction, and the exclusion of other causes of allergy); non allergic (had not eaten any fish 12 hours before the onset of the symptoms); doubtful (who did not remember the previous ingestion of fish or for whom the interval between ingestion and onset of symptoms was between 4 and 12 hours) |
61 overall (25 allergic; 16 non allergic; 20 doubtful) |
92.0% allergic; 50.0% non allergic; 70.0% doubtful |
CAP-radioimmunoassay 100% 3 overall; 100% 3 allergic; 100% 3 non allergic; 100% 3 doubtful |
Immunoblot 1: allergic: 80.0% type 1 pattern, 8.0% type 3; non allergic: 12.5% (n = 2) type 1, 56.3% (n = 9) type 4, 19.0% type 3; doubtful: 40.0% type 1, 35.0% type 3 |
| Gomez, 1998 | Suspected allergy | 147 | 10.0% 2 | 10.0% 2 | - |
| González de Olano, 2007 | Mastocytosis: adults (18–65 years); children (7 months-14 years) |
163 adults; 47 children | - | 26.9% (n = 44) adults; 0.0% (n = 0) children | symptoms referred 13.6% (n = 6) adults; 0.0% (n = 0) children |
| Gonzalez Munoz, 2005 | Suspect allergy subdivided into: Anisakis allergy; chronic urticaria or abdominal pain unrelated to fish ingestion; healthy controls |
88 overall; 37
Anisakis allergy; 51 chronic urticaria or abdominal pain unrelated to fish ingestion; 12 healthy controls |
- |
42.0% (n = 37) had a clinical history of A. simplex allergy confirmed by IgE+ |
CD63 BAT Anisakis+ vs Anisakis- and Anisakis + vs healthy controls, the cutoff for a positive basophil activation test was 21% (specificity = 96%, sensitivity = 100%), and 16% (sensitivity and specificity of 100%) respectively |
| Heffler, 2016 | Allergic clinic outpatients | 3,419 | 15.0% | - | 0.8% + allergic symptoms after raw fish |
| Kimura, 1999 | Urticaria or food allergy | 2,108 | - | 29.8% (n = 629) (>0.7 kU/L IgE) | - |
| Lin, 2012 | Serum samples from Allergy laboratory: sample A without any additional information on analytical results; sample B Phadiatop+ subjects |
600 sample A; 198 sample B | - | ImmunoCAP (> 0.35 kU/L); 2.2% sample A; 6.6% sample B | - |
| Lin, 2014 | Subjects with total IgE levels ≥1000 kU/L | 414 | - | 16.2% (0.2% ELISA with rAni s 1 and rAni s 7) | Immunoblot five bands ranging between 40 and 100 kDa to A. simplex CE |
| Montoro, 1997 | Patients with acute recidivous urticaria who usually eat fish or other seafood. | 25 | 64.0% (n = 16) | 76.0% (n = 19) | Immunoblot 56.0% (14 of the 25) tested sera showed the characteristic band at 49.8–80 kDa compared to the E17 reference serum. Most of the sera showed a common immunorecognition pattern with a group of bands at 200–80 kDa |
| Pascual, 1996 | Patients with increased levels of serum total IgE divided into: shellfish allergy; fish allergy; probable parasitic disease; respiratory allergy |
73 overall; 16 shellfish allergy; 20 fish allergy; 17 probable parasitic disease; 20 respiratory allergy |
- |
56.2% (n = 41) overall; 81.3% shellfish allergy; 40.0% fish allergy; 58.8% probable parasitic disease; 50.0% respiratory allergy |
- |
| Puente, 2008 | Allergic residents with negative skin prick test to Anisakis | 86 | 0.0% 3 | 3.5% UA3 Ani s 7 | - |
| Rodriguez, 2000 | Drug allergic patients | 53 | 54.7% (n = 29) | - | - |
| Ventura, 2013 | Chronic urticaria | 213 | 49.7% | - | - |
| Hospital presenting patients for any reason (5 studies) | |||||
| Andreu-Ballester, 2008 | Non appendectomized controls presenting at emergency department | 80 | - |
1.3% IgG+; 7.5% IgM+; 3.8% IgA+; 5.0% IgE+ |
- |
| Falcao, 2008 | Controls selected for programmed orthopaedic, maxillofacial, or general surgery |
200 (6–18 years) | 5.5% | 1.5% (>0.7 kU/L IgE); 3.0% (>0.35 kU/L IgE) | Combinations SPT ± IgE: 0.5% (SPT+ and >0.7 kU/L IgE); 1.5% (SPT+ and >0.35 kU/L IgE); 6.5% (SPT+ or > 0.7 kU/L IgE); 7.0% (SPT or >0.35 kU/L IgE) |
| Kim 2011 | Subjects presenting at hospital for routine controls | 498 | - |
5.0% larval Anisakis crude extract; 6.6% excretory-secretory proteins |
Immunoblot A specific protein band of 130 kDa was detected from 10 patients with western blot analysis against crude extract and excretory-secretory proteins among those who showed positive results by ELISA |
| Puente, 2008 | Non-digestive nonallergic pathologies unrelated to anisakiosis |
50 | - | 16.0% UA3 Ani s 7 | - |
| Uga, 1996 | Diarrhea /routine check-up without symptoms | 244 | - | 11.0% | - |
| Patients with digestive system disorders (6 studies) | |||||
| Andreu-Ballester,2008 | Cases appendectomized | 80 | - |
2.5% IgG+; 2.5% IgM+; 1.3% IgA+; 2.5% IgE+; |
- |
| Garcia-Perez, 2015 | Cases gastrointestinal cancer | 47 | - | 38.3% IgA1+, rAni s 1, 42.6% IgA1+, rAni s 5 | - |
| Gomez, 1998 | Sample A: eosinophilic gastroenteritis; sample B: digestive disorder different from eosinophilic gastroenteritis | Sample A: 10; Sample B: 10 | Sample A: 80.0%3 Sample B: 10.0%3 | Sample A: 80.0%3; Sample B: 10.0%3 | - |
| Guillen Bueno, 1999 | Crohn disease | 73 | - |
29.0% specific total Ig (G+M+A) 44.0% IgG+; 18.0% IgM+; 53.0% IgA+ |
Immunoblot: human anisakidosis reference serum (E17); 50 and 250 kDa, with a band of about 70 kDa |
| Gutierrez, 2002 | 19 digestive Haemorrhaging; 30 Crohn’s disease; 4 digestive cancer; 5 appendicitis |
57 (42.38 ± 17.60 years) | - |
Crude Extract: Igs-CE 89.4%; IgG-CE 75.4%; IgM- CE 26.3%; IgA- CE 63.1%; IgE- CE 14.0%; Excretory- Secretory antigens: Igs- ES 49.1%; IgG- ES 57.8%; IgM- ES 22.8%; IgA- ES 57.8% |
Immunoblot 24.0% and 48.0% of sera from patients with symptoms of Crohn’s disease and digestive haemorrhaging, respectively, showed a positive immunorecognition pattern of CE antigen. |
| Toro, 2004 | Dyspeptic symptoms | 174 | - |
13.8% (n = 24) IgE anti Ani s 1 |
- |
| Post-partum women (1 study) | |||||
| Figueiredo, 2015 | 170 from high-risk birth unit and 139 from a low-risk birth unit | 309 | - | 19.4% (n = 60) IgG+ | - |
1 Pattern types: type 1: group of several bands of medium molecular weight (MW) (30 to 50 kd) and others of low MW (14 to 30 kd); type 2: two or more bands of medium MW; type 3: only one band of medium MW (about 40 kd); type 4: negative blot without any band.
2 It is not specified whether each subject was tested with both IgE detection and SPT or only one diagnostic technique.
3 The prevalence rate is the result of an inclusion criterion of the study.
Indirect ELISA, ImmunoCAP or RAST were employed in 14 studies on general asymptomatic population; 2 studies among fishing sector workers; 18 studies including symptomatic allergic patients; 5 studies on patients admitted to hospital for any reason; 6 studies on patients with digestive disorders; and 1 study on post-partum women. Two variations of indirect ELISA, that are antigen capture ELISA [47, 35] and rAni s 1, rAni s 7 ELISA [30, 36, 42], were used, respectively, in 5 studies in the general population and in allergic patients.
Immunoblotting technique was performed along with IgE detection by means of previously cited tests in 4 studies on general asymptomatic population [30, 14, 48, 49]; 4 studies on symptomatic population with allergies to any kind of allergen [14, 30, 31,50]; 1 study on patients presenting to hospital for controls [51] and 2 studies on patients with digestive system disorders [32, 49].
General asymptomatic population was investigated for Anisakis sensitization through SPTs assessment in 4 studies (in 1 case it was the only diagnostic criterion employed [52]; in the remaining 3 studies, SPTs were performed along with other IgE detection techniques or CD63 BAT) [14, 38,40].
Occupationally exposed workers were assessed for Anisakis sensitization by SPTs alone in 1 study [5], and by both SPTs and RAST in another one [40].
Cutaneous reactivity to Anisakis extract among allergic patients was also evaluated in 15 studies, including 10 studies which performed both SPTs and IgE detection through indirect ELISA, ImmunoCAP or radioimmunoassays; SPTs and Immunoblot were present in 3 studies protocols; anamnesis of allergic symptoms correlated to fish consumption was considered along with SPTs in 4 studies; finally, Frezzolini et al. [38] added CD63 BAT to SPTs assessment.
Basophil activation test (BAT) was introduced by Gonzalez-Munoz et al. in 2005 [53] and later used by Frezzolini et al. [38] among allergic patients and healthy controls.
Falcao et al. assessed Anisakis sensitization considering the combination of both SPTs and ImmunoCAP positivity in allergic patients and in controls selected for surgery procedures [54]. Similarly, a combination of SPTs and IgE detection was performed by Gomez et al. among digestive disorders patients [55].
General asymptomatic population resulted sensitized to Anisakis in 0.4 to 27.4% of cases by means of indirect ELISA or ImmunoCAP specific IgE detection [14, 35], and between 6.6% and 19.6% of the samples by means of SPTs [14, 38, 40, 52]. Anisakis antigens recognition patterns were obtained by Immunoblotting assays in 25% [14] to 67.5% of sera from asymptomatic general population samples [48].
Occupationally exposed workers (fishermen, fishmongers and workers of fish-processing industries) had specific IgE between 11.7% [4] and 50% of cases [40], whereas SPTs positivity ranged between 8% and 46.4% [5, 40].
Symptomatic allergic patients to any kind of allergen were found to be positive to Anisakis specific IgE detection between 0.0% in children with mastocytosis (González de Olano 2007)[56] to 81.3% among adults with shellfish allergy (Pascual 1996) [44]; diagnostic bands at Immunoblot were visualized in 15–56% of cases (Caballero 2012)[31] (Montoro 1997)[50]. SPT positivity among allergic individuals (14 studies) ranged from 4.5% out of 10570 suspected allergy patients (Consortium 2011) [21] to 64% among 16 patients with acute recidivous urticaria, usually eating fish or other seafood (Montoro 1997) [50]. In particular, chronic urticaria patients reacted to skin tests between 14% and 63% (del Pozo 1997) [57] (Frezzolini A 2010)[38]. The SPT detected also a 14% prevalence of Anisakis positivity among atopic subjects (Frezzolini A 2010) [38], while it estimated a positivity ranging from 4.5% to 15% in patients presenting to allergological clinics to deal with suspected allergy (Consortium 2011) [21] (Heffler E 2016) [58]. When considering also anamnestic criteria (symptoms after fish eating), allergy to Anisakis was found between in 0.0–14.0% of patients [21, 56–58].
Sensitization rates in 5 study-samples selected from hospital-admitted subjects varied according to different criteria to define AS allergy, from 0.5% with a combination of both positive SPTs and >0.7 kU/L IgE (Falcão H 2008) [54] to 20% when IgE >0.35 kU/L were sufficient to be considered positive (Daschner A 1998) [59].
Six other studies investigated the seroprevalence of specific antibodies against Anisakis in patients with digestive system disorders (dyspepsia, appendicits/appendectomized, digestive haemorrage, gastric neoplasms), ranging from 1.3% positive to IgA (Andreu-Ballester JC 2008) [60] to 75.4% for IgG (Gutiérrez R 2002) [32]. Gomez et al. detected 80% of eosinophilic gastroenteritis (EG) patients positive to Anisakis SPTs, but only 10% among subjects who suffered from digestive disorders other than EG [55].
Positive immunorecognition pattern of Anisakis crude extracts (CE) antigens were found in 24% of sera from patients with symptoms of Crohn’s disease and 48% of those with digestive haemorrhaging [32].
Finally, IgG positivity was detected in 19.6% of a sample of postpartum women in Brazil (Figueiredo 2015) [61].
Discussion
We identified 248 research articles and abstracts after searching various bibliographic databases and grey literature. Forty-one studies comprising 31,701 participants from eleven countries overall were included for qualitative synthesis. Most of the studies were set in high raw fish consuming countries, including Spain (n = 22, 6,734 participants) and Italy, where the largest study samples came from (8 studies comprising 17,059 participants). Also, 2 studies took place in Brazil and Norway, respectively, while 1 study was performed in each one of the following countries: Croatia, Indonesia, Japan, Morocco, Portugal, South Africa and South Korea. All the previous evidences support for a global spread of the investigated health subject.
Indirect ELISA and ImmunoCAP methods resulted the most common techniques used to assess Anisakis sensitization by far, measuring the presence of different classes of antibodies against various Anisakis allergens. As expected, higher hypersensitivity rates were obtained from selected samples of symptomatic, allergic subjects usually eating raw or undercooked seafood, coherently with the well-known association between Anisakis sensitization, urticaria/allergic symptoms and undercooked fish intake [44, 50], while prevalence rates tended to be lower if the study sample size was larger [30,62], and when diagnostic techniques were targeting fewer but more specific Anisakis antigens, or when setting higher positivity threshold for specific antibodies detection.
The results of the studies investigating Anisakis sensitization among the general asymptomatic population clearly highlighted the association between fish consumption and Anisakis sensitization. Particularly, the two studies with the largest sample size of random healthy subjects, investigated by SPTs and IgE detection, measured Anisakis responsiveness in 16% out of 187 individuals [52] and 6% out of 1,008, respectively [19]. Prevalence rates were greatly affected by Anisakis antigens chosen as target of diagnostic tests, with large differences between crude extracts of entire Anisakis larvae versus specific recombinant excretory-secretory proteins. More deeply, Anisakis larvae crude extracts (CE) might contain several cross-reactive allergens with other nematodes [63–65], crustaceans, insects or mites [44, 66, 67], and their use as target antigens in commercial assays, both serological (ImmunoCAP) and clinical ones (SPT), may lead to less specificity and consequent overestimation of seroprevalence.
Antigen capture ELISA, a variation of indirect ELISA developed to use recombinant antigens Ani s1 and Ani s7, has been applied by two Spanish studies, showing prevalence ranging from 0.4% out of 2,801 individuals [35] to 11.7% out of 264 adults [47]. Successively, another variation of rAni s1 and rAni s7 indirect ELISA was introduced by Anadon et al. [36] representing the most specific serum test to diagnose anisakiasis, revealing IgE in 40.2% out of 493 allergic subjects in Madrid, with respect to 52.7% positivity prevalence measured by ImmunoCAP from the same serum samples. Antigen capture ELISA with rAni s1 and rAni s7 was latter employed in a Croatian setting, determining 2% out of 500 random healthy subjects sampled from different areas with decreasing prevalence from a maximum of 3.5% among individuals living in islands (assumed as high fish consumers) to 1.5% in urban coastal areas, while a 0.0% prevalence was documented in a rural part of the country (declared to be an area of low or absent seafood intake), stressing the association between Anisakis sensitization and fish intake [42]. Recombinant Ani s1 and rAni s7 indirect ELISA was also used as second-step test to analyse ImmunoCAP positive sera obtained from Norwegian healthy blood donors and selected subjects with >1000 kU/L total IgE, resulting in prevalence rates of 0.0% and 0.2%, respectively, in comparison with 0.4% and 16.2% ImmunoCAP positivity rates from the same samples. It is not clear whether these findings confirm that significant part of the ImmunoCAP positive sera are false-positive due to cross-sensitization, or due to the unspecific binding of very high total IgE levels, or due to minor presence of Anisakis antigens other than rAni s1 and s7 [30]. Similar considerations regarding cross-reactivity issues and not univocal diagnostic criteria apply to the 24 studies performed in subjects with immune disorders or presenting to allergology units to rule out suspected allergy. Findings of these studies associated Anisakis sensitization to both relapsing acute [54] and chronic urticaria [47, 11]; furthermore, allergic manifestation after ingestion of contaminated raw or marinated fish were more frequent when patients were co-sensitized to house dust mites or molds according to SPTs, suggesting possible cross-reactive but clinically relevant allergens between these allergenic sources [58].
Generally, SPTs against Anisakis crude extracts resulted in wide ranges of positivity prevalence: the two largest studies measured 4.5% SPT+ out of 10570 suspected allergy subjects [21] and 15% SPT+ out of 3,410 allergy clinic outpatients [58], both percentages decreased to 0.6% and 0.8%, respectively, when allergic symptoms after raw fish intake was added as diagnostic criterion, suggesting how anamnesis plays an important role in pointing out real allergy versus possible cross-reactivity. SPT positivity without clinical manifestation can still be considered an alarm for future allergic reactions after contact with responsible antigens.
As for IgE detection, the largest Japanese study among 2,108 sera of urticaria or food allergy patients revealed 29.8% seroprevalence with a positivity threshold set at >0.7 kU/L, showing that patients suffering from type I allergic symptoms following ingestion of Anisakis parasitized fishes are more often sensitized to Anisakis specific allergen than to allergens of the seafood per se [20].
Detection of Anisakis-induced basophil activation (BAT) by flow cytometry was introduced by Gonzalez-Munoz et al. in 2005 [53]. Frezzolini et al. [38] latter on compared BAT with SPT and ImmunoCAP in diagnosing Anisakis sensitization among chronic urticaria patients, atopic subjects and healthy controls. All three tests had good similar sensitivity, but highest specificity of 100% was reached only by BAT supporting BAT as reliable diagnostic tool for anisakiasis, resulting in sensitization prevalence of 67% among chronic urticaria patients and 0% among healthy subjects.
Prevalence of detectable antibodies against Anisakis in six studies on patients with anamnesis of digestive disorders (dyspepsia, appendicitis, digestive haemorrhaging, Crohn’s disease, digestive cancer) ranged between 1.3% and 89.4% [32, 60]. However, most studies were of limited sample-size, therefore, no conclusive statement could be drawn in relation of Anisakis sensitization and the reported conditions. Largest sample included 174 dyspeptic patients showing IgE anti Ani s1 in 13.8% of cases. This finding suggests that Anisakis infection might be more frequent than expected, since only the most severe cases that require urgent upper endoscopy examination are being diagnosed at present, and because of confounding clinical manifestations with other conditions. Furthermore, uncooked-fish ingestion and previous gastric surgery were confirmed to be significantly associated with seropositivity for specific IgE against Ani s1 antigen by means of immunoblotting [68].
Although case-control studies alone are not sufficient to assess causality relationships, the significant higher ratio of positivity to secretory IgA1, rAni s1, or rAni s5 found by Garcia-Perez et al. in 47 gastric cancer patients as compared to 47 healthy controls (38.3% vs 6.4%, p-value <0.001 and 42.6% vs 10.6%, p-value <0.001, respectively), together with the evidence that some parasites inducing chronic inflammation may trigger cancer, and that Anisakis larvae have been co-localised incidentally in cases of gastro-intestinal tumours, could suggest that Anisakis infection might be a risk factor for the development of digestive tract cancer [33]. Parasites gastrointestinal lesions often mimic ulcers, so that patients diagnosed with digestive bleeding may suffer from unrecognized anisakiasis, explaining the high prevalence of specific antibodies and immunoblot bands of Anisakis reference serum [32]. By contrast, the transitory lower prevalence of anti-Anisakis specific immunoglobulins documented in 80 appendectomized patients was explained by a diminution of immune responses against pathogens caused by the resection of an area of the Gut Associated Lymphoid Tissue (GALT) [60], even if these results are questionable.
There are no definitive and clear patterns of bands obtained by immunoblotting assays testing for the presence of specific anti-Anisakis IgE. One possible explanation of large differences in molecular weights of the bands detected by immunoblotting may be the lack of unanimous preparation of Anisakis antigenic extracts and the different blotting conditions, which may vary the number of obtained proteins.
Immunoblotting assays were also used as second-step analysis to rule out cross-reactivity among selected sera which resulted in an already positive to Anisakis at ImmunoCAP or ELISA, with miscellaneous results [30, 31,52]. Most of tested sera were positive to Anisakis crude extracts at Immunoblot, but recognized patterns of bands were not univocal and not always concordant with the human anisakiasis reference serum (E1) [30, 52].
We have further analysed the studies dealing with occupationally exposed groups of fishermen, fishmongers and fishing industry workers who are in frequent contact with raw fish and consequently with Anisakis larvae [4,5,40]. Larger study samples resulted in lower sensitization prevalence: SPTs were positive in 8% out of 578 fish industry workers according to Nieuwenhuizen et al. [5] versus 46.4% out of only 28 fishermen/fishmongers in Purello-D’Ambrosio study [40]. Anisakis specific IgE were detected in 11.7% out of 94 fish sector workers by means of UniCAP-100 [4] and 50% out of 28 subjects by means of RAST [40], with antibody levels increasing with duration of occupational exposure. Being at higher risk for sensitization, fishing sector workers can represent ideal candidate for screening and development of better diagnostic tools with ameliorated sensitivity and specificity, to be successively extended in the general population.
Even if we did not perform a quantitative metanalysis, all studies which compared prevalence rates between random healthy subjects and suspected allergic or digestive disorders patients or occupationally exposed workers tended to show lower responsiveness in the former group. The wide heterogeneity in study samples characteristics, design, settings, diagnostic techniques and criteria to define Anisakis sensitization or allergy along with the lack of important information in a large number of studies prevented us to summarize data in order to perform a metanalysis of prevalence results.
Also, from our systematic review important weaknesses emerged referring to the quality of studies available from literature. In fact, most of the studies were not conducted on samples representative of the general population, as the sample size was not calculated a priori to accurately infer sensitization prevalence among the population of origin. A random sampling was never performed, being most of the studies conducted on a convenience sample and the response rate almost never reported. Moreover, in the few studies providing complete details on study population, especially random sera samples were often missing any information about subjects’ gender and age.
Importantly, not all studies specified target antigens of ELISA and UniCAP methods, giving only general information about specific anti-Anisakis IgE detection.
For the previous reasons, comparisons to rule out cross-reactivity influence or differences in specificity and sensitivity were not possible.
Furthermore, correlation between anisakiasis prevalence among different countries with fish parasitism rates of surrounding waters is not straightforward, as nowadays global trading makes seafood from very distant areas easily available. However, high fish consuming habits and genetic susceptibility linked to the presence of DRB1*1502-DQB1*0601 haplotype [69] could partially explain the widespread geographical variety observed [21,42,68].
Low sensitization prevalence among Norwegian blood donors and subjects with >1,000 kU/L total IgE despite frequent seafood intake can be explained by the absence of genetical susceptibility haplotype and by the consumption of mainly processed, canned, frozen and farmed Atlantic salmon (which was demonstrated not to be infected from anisakid nematodes) [30,70].
Lastly, as confirmed by several authors, Anisakis sensitization can be induced by ingestion of well-cooked contaminated fish due to thermo- and pepsin-resistant allergens [54, 59,71, 72], showing a residual allergenic activity also after specific heat treatment [73].
Conclusion
This systematic review has highlighted the epidemiological impact of Anisakis as hypersensitivity aetiologic factor in the general population from several countries worldwide, also with regard to specific groups of patients and occupationally exposed subjects. We observed that hypersensitivity prevalence estimates varied widely according to geographical area, characteristics of the population studied, diagnostic criteria and laboratory assays with varying sensitivity and specificity. Our findings made us conclude that, if, on one hand, Anisakis represents a hidden cause of many adverse reactions after eating undercooked seafood, which are often claimed to be “fish allergy”, including chronic idiopathic urticaria, on the other hand, further studies are needed to overcome the documented misdiagnosis by improving the diagnostic approach and, consequently, to provide more affordable estimates in order to address public health interventions on populations at high risk of exposure to Anisakis and to tailor health services related to specific groups.
Supporting information
(ZIP)
(DOC)
(DOCX)
Abbreviations
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- AS
Anisakis
- ELISA
enzyme-linked immunosorbant assay
- MeSH
Medical Subject Headings
- SPT
Skin prick test
- RAST
Radio-Allergo-Sorbent Test
- OD
optical density
- BAT
Basophil activation test
- EG
eosinophilic gastroenteritis
- CE
crude extracts
- ES
excretory-secretory
- GALT
Gut Associated Lymphoid Tissue
Data Availability
Since the study reported in the manuscript is a Systematic Review (SR), only data already published, without any ethical or legal restriction, have been presented. The final database with the results of the SR is available in the Supporting Information files.
Funding Statement
No funding received.
References
- 1.Anibarro B, Seoane FJ, Mugica MV. Involvement of hidden allergens in food allergic reactions. J Investig Allergol Clin Immunol. 2007. January 1;17(3):168 [PubMed] [Google Scholar]
- 2.EFSA Panel on Biological Hazards (BIOHAZ); Scientific Opinion on risk assessment of parasites in fishery products. EFSA Journal 2010; 8(4):1543 [91 pp.]. 10.2903/j.efsa.2010.1543 Available online: www.efsa.europa.eu. [DOI] [Google Scholar]
- 3.Polimeno L, Loiacono M, Pesetti B, Mallamaci R, Mastrodonato M, Azzarone A et al. Anisakiasis, an Underestimated Infection: Effect on Intestinal Permeability of Anisakis simplex–Sensitized Patients. Foodborne Pathog Dis. 2010. July 1;7(7):809–14. 10.1089/fpd.2009.0484 [DOI] [PubMed] [Google Scholar]
- 4.Mazzucco W, Lacca G, Cusimano R, Provenzani A, Costa A, Di Noto AM et al. Prevalence of sensitization to Anisakis simplex among professionally exposed populations in Sicily. Arch Environ Occup Health. 2012. April 1;67(2):91–7. 10.1080/19338244.2011.578683 [DOI] [PubMed] [Google Scholar]
- 5.Nieuwenhuizen N, Lopata AL, Jeebhay MF, De'Broski RH, Robins TG, Brombacher F. Exposure to the fish parasite Anisakis causes allergic airway hyperreactivity and dermatitis. J Allergy Clin Immunol. 2006. May 31;117(5):1098–105. 10.1016/j.jaci.2005.12.1357 [DOI] [PubMed] [Google Scholar]
- 6.Nieuwenhuizen NE, Lopata AL. Allergic reactions to Anisakis found in fish. Curr Allergy Asthma Rep. 2014. August;14(8):455 10.1007/s11882-014-0455-3 [DOI] [PubMed] [Google Scholar]
- 7.Nieuwenhuizen NE. Anisakis–immunology of a foodborne parasitosis. Parasite immunol. 2016. September 1;38(9):548–57. 10.1111/pim.12349 [DOI] [PubMed] [Google Scholar]
- 8.Hochberg NS, Hamer DH, Hughes JM, Wilson ME. Anisakidosis: perils of the deep. Clin Infect Dis. 2010. October 1;51(7):806–12. 10.1086/656238 [DOI] [PubMed] [Google Scholar]
- 9.Daschner A, Alonso-Gómez A, Cabañas R, Suarez-de-Parga JM, López-Serrano MC. Gastroallergic anisakiasis: borderline between food allergy and parasitic disease—clinical and allergologic evaluation of 20 patients with confirmed acute parasitism by Anisakis simplex. J Allergy Clin Immunol. 2000. January 31;105(1):176–81. [DOI] [PubMed] [Google Scholar]
- 10.Audicana MT, Kennedy MW. Anisakis simplex: from obscure infectious worm to inducer of immune hypersensitivity. Clin microbiol rev. 2008. April 1;21(2):360–79. 10.1128/CMR.00012-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daschner A, Pascual CY. Anisakis simplex: sensitization and clinical allergy. Curr Opin Allergy Clin Immunol. 2005. June;5(3):281–5. Review. [DOI] [PubMed] [Google Scholar]
- 12.Choi SJ, Lee JC, Kim MJ, Hur GY, Shin SY, Park HS. The clinical characteristics of Anisakis allergy in Korea. Korean J Intern Med. 2009. June;24(2):160–3. 10.3904/kjim.2009.24.2.160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kasuya S, Hamano H, Izumi S. Mackerel-induced urticaria and Anisakis. Lancet. 1990. March 17;335(8690):665 [DOI] [PubMed] [Google Scholar]
- 14.García M, Moneo I, Audicana MT, del Pozo MD, Muñoz D, Fernández E et al. The use of IgE immunoblotting as a diagnostic tool in Anisakis simplex allergy. J Allergy Clin Immunol.1997. April 30;99(4):497–501. 10.1016/S0091-6749(97)70076-9. [DOI] [PubMed] [Google Scholar]
- 15.Lorenzo S, Iglesias R, Leiro J, Ubeira FM, Ansotegui I, García M et al. Usefulness of currently available methods for the diagnosis of Anisakis simplex allergy. Allergy. 2000;55(7):627–33. [DOI] [PubMed] [Google Scholar]
- 16.Sastre J, Lluch‐Bernal M, Quirce S, Arrieta I, Lahoz C, Del Amo A, et al. A double blind, placebo-controlled oral challenge study with lyophilized larvae and antigen of the fish parasite, Anisakis simplex. Allergy. 55:560–564. [DOI] [PubMed] [Google Scholar]
- 17.Lluch-Bernal M, Sastre J, Fernández-Caldas E, Marañon F, Cuesta-Herranz J, De las Heras M et al. Conjunctival provocation tests in the diagnosis of Anisakis simplex hypersensitivity. J Investig Allergol Clin Immunol. 2002;12(1):21–4. [PubMed] [Google Scholar]
- 18.Miles S, Fordham R, Mills C, Valovirta E, Mugford M. A framework for measuring costs to society of IgE-mediated food allergy. Allergy. 2005;60:996–1003. 10.1111/j.1398-9995.2005.00868.x [DOI] [PubMed] [Google Scholar]
- 19.García-Palacios L, González ML, Esteban MI, Mirabent E, Perteguer MJ, Cuéllar C. Enzyme-linked immunosorbent assay, immunoblot analysis and RAST fluoroimmunoassay analysis of serum responses against crude larval antigens of Anisakis simplex in a Spanish random population. J Helminthol. 1996. December;70(4):281–9. [DOI] [PubMed] [Google Scholar]
- 20.Kimura S, Takagi Y, Gomi K. IgE response to Anisakis compared to seafood. Allergy. 1999. November 1;54(11):1225–6. [DOI] [PubMed] [Google Scholar]
- 21.AAITO-IFIACI Anisakis Consortium. Anisakis hypersensitivity in Italy: prevalence and clinical features: a multicenter study. Allergy. 2011. December 1;66(12):1563–9. 10.1111/j.1398-9995.2011.02691.x [DOI] [PubMed] [Google Scholar]
- 22.Armentia A, Lombardero M, Callejo A, Martin Santos JM, Gil FJ, Vega J, et al. Occupational asthma by Anisakis simplex. J Allergy Clin Immunol. 1998;102:831–4. [DOI] [PubMed] [Google Scholar]
- 23.Scala E, Giani M, Pirrotta L, Guerra EC, Cadoni S, Girardelli CR, et al. Occupational generalised urticaria and allergic airborne asthma due to Anisakis simplex. Eur J Dermatol. 2001;11:249–50. [PubMed] [Google Scholar]
- 24.Barbuzza O, Guarneri F, Galtieri G, Gangemi S, Vaccaro M. Protein contact dermatitis and allergic asthma caused by Anisakis simplex. Contact Dermatitis. 2009: 60: 239–240. 10.1111/j.1600-0536.2009.01519.x [DOI] [PubMed] [Google Scholar]
- 25.Conde-Salazar L, Gonzalez MA, Guimaraens D. Type I and Type IV sensitization to Anisakis simplex in 2 patients with hand eczema. Contact Dermatitis. 2002;46:36. [DOI] [PubMed] [Google Scholar]
- 26.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009 Oct;62(10):1006–12. 10.1016/j.jclinepi.2009.06.005 Epub 2009 Jul 23. [DOI] [PubMed] [Google Scholar]
- 27.Munn Z, Moola S, Riitano D, Lisy K. The development of a critical appraisal tool for use in systematic reviews addressing questions of prevalence. Int J Health Policy Manag. 2014; 3(3):123–8. doi: 10.15171/ijhpm.2014.71 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gómez B, Tabar AI, Tuñón T, Larrínaga B, Alvarez MJ, García BE, et al. Eosinophilic gastroenteritis and Anisakis. Allergy. 1998. December;53(12):1148–54. [DOI] [PubMed] [Google Scholar]
- 29.Desowitz RS, Raybourne RB, Ishikura H, Kliks MM. The radioallergosorbent test (RAST) for the serologic diagnosis of human anisakiasis. Trans R Soc Trop Med Hyg. 1985;79:256–9. [DOI] [PubMed] [Google Scholar]
- 30.Lin AH, Nepstad I, Florvaag E, Egaas E, Van Do T. An extended study of seroprevalence of anti-Anisakis simplex IgE antibodies in Norwegian blood donors. Scand J Immunol. 2014. January;79(1):61–7. 10.1111/sji.12130 [DOI] [PubMed] [Google Scholar]
- 31.Caballero ML, Umpierrez A, Perez-Piñar T, Moneo I, de Burgos C, Asturias JA, et al. Anisakis simplex recombinant allergens increase diagnosis specificity preserving high sensitivity. Int Arch Allergy Immunol. 2012;158(3):232–40. 10.1159/000331581 Epub 2012 Mar 2. [DOI] [PubMed] [Google Scholar]
- 32.Gutiérrez R, Cuéllar C. Immunoglobulins anti-Anisakis simplex in patients with gastrointestinal diseases. J Helminthol. 2002. June;76(2):131–6. 10.1079/JOH2001104 [DOI] [PubMed] [Google Scholar]
- 33.Garcia-Perez JC, Rodríguez-Perez R, Ballestero A, Zuloaga J, Fernandez-Puntero B, Arias-Díaz J, et al. Previous Exposure to the Fish Parasite Anisakis as a Potential Risk Factor for Gastric or Colon Adenocarcinoma. Medicine (Baltimore). 2015. October;94(40):e1699 10.1097/MD.0000000000001699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Figueiredo Junior I, Vericimo MA, Cardoso LR, São Clemente SC, do Nascimento ER, Teixeira GA. Cross-sectional study of serum reactivity to Anisakis simplex in healthy adults in Niterói, Brazil. Acta Parasitol. 2013 Sep;58(3):399–404. 10.2478/s11686-013-0157-3 Epub 2013 Aug 29. [DOI] [PubMed] [Google Scholar]
- 35.Valiñas B, Lorenzo S, Eiras A, Figueiras A, Sanmartín ML, Ubeira FM. Prevalence of and risk factors for IgE sensitization to Anisakis simplex in a Spanish population. Allergy. 2001. July;56(7):667–71. [DOI] [PubMed] [Google Scholar]
- 36.Anadón AM, Rodríguez E, Gárate MT, Cuéllar C, Romarís F, Chivato T, et al. Diagnosing human anisakiasis: recombinant Ani s 1 and Ani s 7 allergens versus the UniCAP 100 fluorescence enzyme immunoassay. Clin Vaccine Immunol. 2010. April;17(4):496–502. 10.1128/CVI.00443-09 Epub 2010 Jan 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Del Rey Moreno A, Valero A, Mayorga C, Gómez B, Torres MJ, Hernández J, et al. Sensitization to Anisakis simplex s.l. in a healthy population. Acta Trop. 2006. March;97(3):265–9. Epub 2006 Jan 24. 10.1016/j.actatropica.2005.11.007 [DOI] [PubMed] [Google Scholar]
- 38.Frezzolini A, Cadoni S, De Pità O. Usefulness of the CD63 basophil activation test in detecting Anisakis hypersensitivity in patients with chronic urticaria: diagnosis and follow-up. Clin Exp Dermatol. 2010. October;35(7):765–70. 10.1111/j.1365-2230.2009.03694.x [DOI] [PubMed] [Google Scholar]
- 39.Bernardini R, Lombardi E, Novembre E, Ingargiola A, Pucci N, Favilli T, et al. Predictors of Anisakis simplex symptoms. Allergy. 2000. October 1;55(10):979–80. [DOI] [PubMed] [Google Scholar]
- 40.Purello-D'Ambrosio F, Pastorello E, Gangemi S, Lombardo G, Ricciardi L, Fogliani O, et al. Incidence of sensitivity to Anisakis simplex in a risk population of fishermen/fishmongers. Ann Allergy Asthma Immunol. 2000. April;84(4):439–44. 10.1016/S1081-1206(10)62278-8 [DOI] [PubMed] [Google Scholar]
- 41.Abattouy N, Valero A, Martín-Sánchez J, Peñalver MC, Lozano J. Sensitization to Anisakis simplex species in the population of northern Morocco. J Investig Allergol Clin Immunol. 2012;22(7):514–9. [PubMed] [Google Scholar]
- 42.Mladineo I, Poljak V, Martínez-Sernández V, Ubeira FM. Anti-Anisakis IgE seroprevalence in the healthy Croatian coastal population and associated risk factors. PLoS Negl Trop Dis.2014. 2014 February 6;8(2):e2673 10.1371/journal.pntd.0002673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Estrada Rodríguez JL, Gozalo Reques F. Sensitization to Anisakis simplex: an unusual presentation. Allergol Immunopathol (Madr). 1997. Mar-Apr;25(2):95–7. [PubMed] [Google Scholar]
- 44.Pascual C, Crespo JF, Ortega N, Ornia N, San-Martin MS, Martin-Esteban M. High prevalence of sensitization to Anisakis simplex (AK) in patients with increased levels of total IgE. J Allergy Clin Immunol. 1996. January 1;97(1):233. [Google Scholar]
- 45.Rodriguez A, Trujillo MJ, Tornero P, Baeza ML, Herrero T, Pérez A, et al. 1042 Prevalence of anisakis simplex sensitization in patients with suspicion of drug allergy. J Allergy Clin Immunol. 2000. January 1;105(1):S354. [Google Scholar]
- 46.Uga S, Ono K, Kataoka N, Hasan H. Seroepidemiology of five major zoonotic parasite infections in inhabitants of Sidoarjo, East Java, Indonesia. Southeast Asian J Trop Med Public Health.1996. September 1;27:556–61. [PubMed] [Google Scholar]
- 47.Puente P, Anadón AM, Rodero M, Romarís F, Ubeira FM, Cuéllar C. Anisakis simplex: the high prevalence in Madrid (Spain) and its relation with fish consumption. Exp Parasitol. 2008. February;118(2):271–4. Epub 2007 Aug 3. 10.1016/j.exppara.2007.07.002 [DOI] [PubMed] [Google Scholar]
- 48.Del Rey Moreno A, Valero A, Mayorga C, Gómez B, Torres MJ, Hernández J, et al. Sensitization to Anisakis simplex s.l. in a healthy population. Acta Trop. 2006. March;97(3):265–9. 10.1016/j.actatropica.2005.11.007 [DOI] [PubMed] [Google Scholar]
- 49.Guillén-Bueno R, Gutiérrez-Ramos R, Perteguer-Prieto MJ, Olveira-Martin A, Fernandez-Blanco I, Pozuelo-García A, et al. Anti-anisakis antibodies in the clinical course of Crohn's disease. Digestion. 1999;60(3):268–73. 10.1159/000007668 [DOI] [PubMed] [Google Scholar]
- 50.Montoro A, Perteguer MJ, Chivato T, Laguna R, Cuéllar C. Recidivous acute urticaria caused by Anisakis simplex. Allergy. 1997. October;52(10):985–91. [DOI] [PubMed] [Google Scholar]
- 51.Kim J, Jo JO, Choi SH, Cho MK, Yu HS, Cha HJ, et al. Seroprevalence of antibodies against Anisakis simplex larvae among health-examined residents in three hospitals of southern parts of Korea. Korean J Parasitol. 2011. June;49(2):139–44. 10.3347/kjp.2011.49.2.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ventura MT, Napolitano S, Menga R, Cecere R, Asero R. Anisakis simplex hypersensitivity is associated with chronic urticaria in endemic areas. Int Arch Allergy Immunol. 2013;160(3):297–300. 10.1159/000339869 [DOI] [PubMed] [Google Scholar]
- 53.Gonzalez-Muñoz M, Luque R, Nauwelaers F, Moneo I. Detection of Anisakis simplex-induced basophil activation by flow cytometry. Cytometry B Clin Cytom. 2005. November;68(1):31–6. 10.1002/cyto.b.20070 [DOI] [PubMed] [Google Scholar]
- 54.Falcão H, Lunet N, Neves E, Iglésias I, Barros H. Anisakis simplex as a risk factor for relapsing acute urticaria: a case-control study. J Epidemiol Community Health. 2008. July;62(7):634–7. 10.1136/jech.2007.061572 [DOI] [PubMed] [Google Scholar]
- 55.Gómez B, Tabar AI, Tuñón T, Larrínaga B, Alvarez MJ, Garcia BE, et al. Eosinophilic gastroenteritis and Anisakis. Allergy. 1998. December;53(12):1148–5 [DOI] [PubMed] [Google Scholar]
- 56.Gonzalez de Olano D, De La Hoz Caballer B, Nunez Lopez R, Sanchez Munoz L, Cuevas Agustin M, Dieguez MC, et al. Prevalence of allergy and anaphylactic symptoms in 210 adult and pediatric patients with mastocytosis in Spain: a study of the Spanish network on mastocytosis (REMA). Clin Exp Allergy. 2007. October;37(10):1547–55. 10.1111/j.1365-2222.2007.02804.x [DOI] [PubMed] [Google Scholar]
- 57.Del Pozo MD, Audícana M, Diez JM, Munoz D, Ansotegui IJ, Fernández E, et al. Anisakis simplex, a relevant etiologic factor in acute urticaria. Allergy. 1997. May;52(5):576–9. [DOI] [PubMed] [Google Scholar]
- 58.Heffler E, Sberna ME, Sichili S, Intravaia R, Nicolosi G, Porto M, et al. High prevalence of Anisakis simplex hypersensitivity and allergy in Sicily, Italy. Ann Allergy Asthma Immunol. 2016. February;116(2):146–50. 10.1016/j.anai.2015.12.014 [DOI] [PubMed] [Google Scholar]
- 59.Daschner A, Alonso-Gómez A, Caballero T, Barranco P, Suarez-De-Parga JM, López-Serrano MC. Gastric anisakiasis: an underestimated cause of acute urticaria and angio-oedema? Br J Dermatol. 1998. November;139(5):822–8. [DOI] [PubMed] [Google Scholar]
- 60.Andreu-Ballester JC, Pérez-Griera J, Ballester F, Colomer-Rubio E, Ortiz-Tarín I, Pelayo V, et al. Anisakis simplex and Kudoa sp.: evaluation of specific antibodies in appendectomized patients. Exp Parasitol. 2008. July;119(3):433–6. 10.1016/j.exppara.2008.03.014 [DOI] [PubMed] [Google Scholar]
- 61.Figueiredo I, Vericimo M, Terra L, Ferreira T, São Clemente SC, Teixeira G. Association between immunoreactivity to Anisakis spp. antigens and high-risk pregnancy. Acta Parasitol. 2015. Dec;60(4):609–13. 10.1515/ap-2015-0085 [DOI] [PubMed] [Google Scholar]
- 62.Lin AH, Florvaag E, Van Do T, Johansson SG, Levsen A, Vaali K. IgE sensitization to the fish parasite Anisakis simplex in a Norwegian population: a pilot study. Scand J Immunol. 2012. April;75(4):431–5. 10.1111/j.1365-3083.2012.02670.x [DOI] [PubMed] [Google Scholar]
- 63.Kennedy MW, Tierney J, Ye P, McMonagle FA, McIntosh A, McLaughlin D, et al. The secreted and somatic antigens of the third stage larva of Anisakis simplex, and antigenic relationship with Ascaris suum, Ascaris lumbricoides, and Toxocara canis. Mol Biochem Parasitol. 1988. October;31(1):35–46. [DOI] [PubMed] [Google Scholar]
- 64.Iglesias R, Leiro J, Ubeira FM, Santamarina MT, Navarrete I, Sanmartín ML. Antigenic cross-reactivity in mice between third-stage larvae of Anisakis simplex and other nematodes. Parasitol Res. 1996;82(4):378–81. [DOI] [PubMed] [Google Scholar]
- 65.Lorenzo S, Iglesias R, Audícana MT, García-Villaescusa R, Pardo F, Sanmartín ML, et al. Human immunoglobulin isotype profiles produced in response to antigens recognized by monoclonal antibodies specific to Anisakis simplex. Clin Exp Allergy. 1999. August;29(8):1095–101. [DOI] [PubMed] [Google Scholar]
- 66.Johansson E, Aponno M, Lundberg M, van Hage-Hamsten M. Allergenic cross-reactivity between the nematode Anisakis simplex and the dust mites Acarus siro, Lepidoglyphus destructor, Tyrophagus putrescentiae, and Dermatophagoides pteronyssinus. Allergy. 2001. July;56(7):660–6. [DOI] [PubMed] [Google Scholar]
- 67.Guarneri F, Guarneri C, Benvenga S. Cross-reactivity of Anisakis simplex: possible role of Ani s 2 and Ani s 3. Int J Dermatol. 2007. February;46(2):146–50. 10.1111/j.1365-4632.2006.03091.x [DOI] [PubMed] [Google Scholar]
- 68.Toro C, Caballero ML, Baquero M, García-Samaniego J, Casado I, Rubio M, et al. High prevalence of seropositivity to a major allergen of Anisakis simplex, Ani s 1, in dyspeptic patients. Clin Diagn Lab Immunol. 2004. January;11(1):115–8. 10.1128/CDLI.11.1.115-118.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sanchez-Velasco P, Mendizabal L, Anton EM, Ocejo-Vinyals G, Jerez J, Leyva-Cobian F. Association of hypersensitivity to the nematode Anisakis simplex with HLA class II DRB1*1502-DQB1*0601 haplotype. Hum Immunol, 2000;61:314–9. [DOI] [PubMed] [Google Scholar]
- 70.Alexander J, Frøyland L, Hemre GI. Fish and Seafood Consumption in Norway–Benefits and Risks. Norway: Norwegian Scientific Committee for Food Safety, 2006. [Google Scholar]
- 71.Caballero ML, Moneo I. Several allergens from Anisakis simplex are highly resistant to heat and pepsin treatments. Parasitol Res. 2004;93:248–51. 10.1007/s00436-004-1099-3 [DOI] [PubMed] [Google Scholar]
- 72.Vidaček S, de las Heras C, Solas MT, Mendizábal A, Rodriguez‐Mahillo AI, González‐Muñoz M, et al. Anisakis simplex allergens remain active after conventional or microwave heating and pepsin treatments of chilled and frozen L3 larvae. J Sci Food Agric. 2009;89:1997–2002. [Google Scholar]
- 73.Tejada M, Olivares F, de las Heras C, Careche M, Solas MT, García ML, et al. Antigenicity of Anisakis simplex s.s. L3 in parasitized fish after heating conditions used in the canning processing. J Sci Food Agric. 2015. March 30;95(5):922–7. 10.1002/jsfa.6763 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(ZIP)
(DOC)
(DOCX)
Data Availability Statement
Since the study reported in the manuscript is a Systematic Review (SR), only data already published, without any ethical or legal restriction, have been presented. The final database with the results of the SR is available in the Supporting Information files.