Abstract
Background
Low-grade gliomas affect younger adults and carry a favorable prognosis. They include a variety of biological features affecting clinical behavior and treatment. Having no guidelines on treatment established, we aim to describe clinical and treatment patterns of low-grade gliomas across the largest cancer database in the United States.
Methods
We analyzed the National Cancer Database from 2004 to 2015, for adult patients with a diagnosis of World Health Organization grade II diffuse glioma.
Results
We analyzed 13,621 cases with median age of 41 years. Over 56% were male, 88.4% were white, 6.1% were black, and 7.6% Hispanic. The most common primary site location was the cerebrum (79.9%). Overall, 72.2% received surgery, 36.0% radiation, and 27.3% chemotherapy. Treatment combinations included surgery only (41.5%), chemotherapy + surgery (6.6%), chemotherapy only (3.1%), radiation + chemotherapy + surgery (10.7%), radiation + surgery (11.5%), radiation only (6.1%), and radiotherapy + chemotherapy (6.7%). Radiation was more common in treatment of elderly patients, 1p/19q co-deletion (37.3% versus 24.3%, p<0.01), and tumors with midline location. Median survival was 11 years with younger age, 1p/19q co-deletion, and cerebrum location offered survival advantage.
Conclusions
Tumor location, 1p/19q co-deletion, and age were the main determinants of treatment received and survival, likely reflecting tumor biology differences. Any form of treatment was preferred over watchful waiting in the majority of the patients (86.1% versus 8.1%). Survival of low-grade gliomas is higher than previously reported in the majority of clinical trials and population-based analyses. Our analysis provides a real world estimation of treatment decisions, use of molecular data, and outcomes.
Introduction
Low-grade gliomas have a wide variety of histologic and molecular features corresponding to a grade II in the World Health Organization (WHO) Classification of Central Nervous System Tumors[1]. This group includes astrocytomas, oligodendrogliomas, and oligoastrocytomas. However, evidence of molecular genetic analyses demonstrates that the vast majority of tumors previously classified as oligoastrocytomas have a genetic profile typical of either diffuse astrocytoma or oligodendroglioma, with few true cases of oligoastroctyomas[1]. Therefore this category is now designed as NOS in the 2016 classification [2], and its use is expected to decrease.
Low-grade gliomas are most common in young adults between 35 and 44 years of age[3]. They are a slower growing group of tumors, but a subgroup can be fast growing; still their prognosis is favorable compared to high-grade gliomas. However, most low-grade gliomas eventually transform into high-grade gliomas, resulting in debate in determining the first course of treatment, the time and aggressiveness of surgery, and the role of adjuvant treatment. Watchful waiting until progression may be an acceptable option in selected patients, however surgical resection often results in improved outcomes and symptom control, particularly tumor-related epilepsy[4–6], which is of especial interest due to the higher rate of seizures in isocitrate dehydrogenase (IDH) mutant tumors[7].
Since 2016 molecular markers have been included in the classification[1]. This has translated into treatment decisions; presence of IDH mutation, 1p/19q co-deletion, ATRX expression, and TERT promoter mutation are used in diagnosis and provide prognosis estimates[8, 9]. Other factors such as older age (>40 years), incomplete bulk tumor resection, or having an unfavorable molecular profile, such as IDH wild type, are considered in treatment decisions for possible radiation and chemotherapy[10–13]. An established standard of care has not been defined, and treatment strategies often differ among physicians. Our analysis describes the prognosis and therapeutic patterns of care for diffuse gliomas in American College of Surgeons Commission on Cancer (CoC)-accredited hospitals in the United States using the National Cancer Database (NCDB).
Methods
NCDB is a joint program of the American College of Surgeons Commission on Cancer (CoC) and the American Cancer Society that collects cases from over 1,500 CoC-accredited hospitals. With over 34 million cases, it is the largest cancer database in the United States. Data collection methods, characteristics of participating hospitals and patients, and assessment of data quality have been described elsewhere, and has been used extensively in treatment description of primary brain tumors[14, 15].
We used the 2015 NCDB brain/central nervous system participant user file, which includes cases from 2004 to 2015. Institutional Review Board approval was obtained as exception as determined by the University of Kentucky.
Cases of diffuse glioma were identified using the International Classification of Disease for Oncology (ICD-03) histology codes 9380–9382, 9400, 9401, 9410, 9411, 9420, 9421, 9424, 9425, 9431, 9450, 9451, 9460. Patients with diagnosis of WHO grade I, III, IV, or not reported, and patients younger than 18 years were excluded from the analysis.
Demographic variables that were analyzed include age at diagnosis, sex, race, Hispanic origin, primary payer status, median income, education status, and urban/rural residence. Living area, as determined by the zip code of the patient recorded at the time of diagnosis was used to classify patients as urban or rural, as defined by the United States Department of Agriculture Economic Research Service[16]. Charlson-Deyo score[17] was used to assess patient comorbidities and was categorized as 0, 1, 2, or ≥ 3, as available in NCDB. Tumor location was defined as midline (brainstem, spine, and ventricles not otherwise specified), cerebrum (cerebrum and lobes), or other (including meninges, brain, and nervous system not otherwise specified). Education was assessed by the percentage of non-high school graduates in the patient zip code at the time of diagnosis (≤ 7%, 7–12.9%, 13–20.9%, ≥ 21%).
Disease characteristics included in our analyses were primary site location, histology group, chromosome 1p, and chromosome 19q loss of heterozygosity. Histology groups were divided into astrocytic (9400, 9401, 9410, 9411, 9420, 9421, 9424, 9425), oligodendroglial (9450, 9451, 9460), and mixed (9380, 9381, 9382) as NCDB uses the WHO 2007 classification. 1p/19q co-deletion status was available for cases diagnosed after 2010. Co-deletion was defined as having both chromosomal arm deletions as reported in the Collaborative Stage Site-Specific Factors by NCDB, cases negative for both chromosome deletions were defined as negative, and patients with only one deletion reported were considered incomplete. Treatment received was assessed as first course of treatment at any CoC facility, as previously described by our group[18].
Statistical analysis
Chi square was performed for categorical variables. Age groups cutoff were determined based on historical reports[10, 11] and increased mortality cutoffs using area under the curve. Survival and risk of mortality was assessed as previously described by our group using Kaplan Meier and Cox proportional hazards models[18]. The level of statistical significance was set at 0.05 for all tests conducted, and all analyses were performed with SAS software version 9.4 (SAS Statistical Institute, Cary, North Carolina).
Results
A total of 13,621 cases were identified. The median age at diagnosis was 41 years (range: 18–90) and 56% were male. Race was collected for 98.5% of the patients and was grouped in white, black, and others. The most common race was white (88.5%), followed by black (6.1%), and other races (3.9%). Hispanic origin was available for 93.6% of the patients, with 7.5% identified as Hispanic. Hispanic origin was more common in younger age groups. Younger patients had a higher proportion of blacks and other races (Table 1), that may represent demographic trends in the United States, independent of tumor biology. Eighty-six percent had a Charlson-Deyo score (measurement of co-morbidities) of zero, while 1% had a score ≥3. Charlson-Deyo score increased with increasing age but did not vary by histological groups or molecular determinants (Table 2 and Table 3).
Table 1. Characteristics of the study population, by age group.
| 18–40 N = 6656 |
41–60 N = 5113 |
≥60 N = 1852 |
All N = 13621 |
p-value | ||
|---|---|---|---|---|---|---|
| Gender | Male | 3767 (56.6%) | 2890 (56.5%) | 973 (52.5%) | 7630 (56.0%) | < .01 |
| Female | 2889 (43.4%) | 2223 (43.5%) | 879 (47.5%) | 5991 (44.0%) | ||
| Race | White | 5798 (87.1%) | 4568 (89.3%) | 1685 (91.0%) | 12051 (88.5%) | < .01 |
| Black | 438 (6.6%) | 302 (5.9%) | 97 (5.2%) | 837 (6.1%) | ||
| Other | 315 (4.7%) | 166 (3.2%) | 52 (2.8%) | 533 (3.9%) | ||
| Unknown | 105 (1.6%) | 77 (1.5%) | 18 (1.0%) | 200 (1.5%) | ||
| Hispanic | Unknown | 407 (6.1%) | 335 (6.6%) | 126 (6.8%) | 868 (6.4%) | < .01 |
| No | 5644 (84.8%) | 4447 (87.0%) | 1640 (88.6%) | 11731 (86.1%) | ||
| Yes | 605 (9.1%) | 331 (6.5%) | 86 (4.6%) | 1022 (7.5%) | ||
| Tumor location | NOS | 997 (15.0%) | 885 (17.3%) | 376 (20.3%) | 2258 (16.6%) | < .01 |
| Cerebrum | 5430 (81.6%) | 4071 (79.6%) | 1385 (74.8%) | 10886 (79.9%) | ||
| Midline | 229 (3.4%) | 157 (3.1%) | 91 (4.9%) | 477 (3.5%) | ||
| Charlson- | 0 | 6040 (90.7%) | 4311 (84.3%) | 1308 (70.6%) | 11659 (85.6%) | < .01 |
| Deyo | 1 | 456 (6.9%) | 573 (11.2%) | 375 (20.2%) | 1404 (10.3%) | |
| Score | 2 | 130 (2.0%) | 175 (3.4%) | 121 (6.5%) | 426 (3.1%) | |
| ≥3 | 30 (0.5%) | 54 (1.1%) | 48 (2.6%) | 132 (1.0%) | ||
| Facility | Unknown | 6312 (94.8%) | 0 (0.0%) | 0 (0.0%) | 6312 (46.3%) | < .01 |
| Type | Community | 143 (2.1%) | 2252 (44.0%) | 963 (52.0%) | 3358 (24.7%) | |
| Academic | 201 (3.0%) | 2861 (56.0%) | 889 (48.0%) | 3951 (29.0%) | ||
| Living area | Unknown | 215 (3.2%) | 205 (4.0%) | 83 (4.5%) | 503 (3.7%) | < .01 |
| Metro | 5441 (81.7%) | 4075 (79.7%) | 1425 (76.9%) | 10941 (80.3%) | ||
| Rural | 1000 (15.0%) | 833 (16.3%) | 344 (18.6%) | 2177 (16.0%) | ||
| Treatment | Unknown | 413 (6.2%) | 265 (5.2%) | 79 (4.3%) | 757 (5.6%) | < .01 |
| None | 432 (6.5%) | 416 (8.1%) | 261 (14.1%) | 1109 (8.1%) | ||
| RT+CT+S | 604 (9.1%) | 658 (12.9%) | 193 (10.4%) | 1455 (10.7%) | ||
| RT+CT | 280 (4.2%) | 402 (7.9%) | 232 (12.5%) | 914 (6.7%) | ||
| RT+S | 680 (10.2%) | 654 (12.8%) | 235 (12.7%) | 1569 (11.5%) | ||
| CT+S | 426 (6.4%) | 393 (7.7%) | 77 (4.2%) | 896 (6.6%) | ||
| S only | 3397 (51.0%) | 1797 (35.1%) | 464 (25.1%) | 5658 (41.5%) | ||
| RT only | 243 (3.7%) | 343 (6.7%) | 248 (13.4%) | 834 (6.1%) | ||
| CT only | 181 (2.7%) | 185 (3.6%) | 63 (3.4%) | 429 (3.1%) | ||
| Insurance status | Not Insured | 585 (8.8%) | 322 (6.3%) | 40 (2.2%) | 947 (7.0%) | < .01 |
| Private | 4636 (69.7%) | 3939 (77.0%) | 570 (30.8%) | 9145 (67.1%) | ||
| Medicaid | 998 (15.0%) | 420 (8.2%) | 51 (2.8%) | 1469 (10.8%) | ||
| Medicare | 162 (2.4%) | 227 (4.4%) | 1144 (61.8%) | 1533 (11.3%) | ||
| Other Gov’t | 140 (2.1%) | 95 (1.9%) | 19 (1.0%) | 254 (1.9%) | ||
| Unknown | 135 (2.0%) | 110 (2.2%) | 28 (1.5%) | 273 (2.0%) |
NOS: Not otherwise specified; RT: Radiation therapy; CT: Chemotherapy; S: Surgery
Table 2. Characteristics of the study population, by histology group.
| Astrocytic N = 6050 |
Mixed N = 2795 |
Oligodendroglial N = 4776 |
All N = 13621 |
p-value | ||
|---|---|---|---|---|---|---|
| Gender | Male | 3381 (55.9%) | 1593 (57.0%) | 2656 (55.6%) | 7630 (56.0%) | 0.49 |
| Female | 2669 (44.1%) | 1202 (43.0%) | 2120 (44.4%) | 5991 (44.0%) | ||
| Race | White | 5307 (87.7%) | 2479 (88.7%) | 4265 (89.3%) | 12051 (88.5%) | < .01 |
| Black | 434 (7.2%) | 161 (5.8%) | 242 (5.1%) | 837 (6.1%) | ||
| Other | 223 (3.7%) | 109 (3.9%) | 201 (4.2%) | 533 (3.9%) | ||
| Unknown | 86 (1.4%) | 46 (1.6%) | 68 (1.4%) | 200 (1.5%) | ||
| Hispanic | Unknown | 355 (5.9%) | 202 (7.2%) | 311 (6.5%) | 868 (6.4%) | 0.14 |
| No | 5234 (86.5%) | 2378 (85.1%) | 4119 (86.2%) | 11731 (86.1%) | ||
| Yes | 461 (7.6%) | 215 (7.7%) | 346 (7.2%) | 1022 (7.5%) | ||
| Tumor location | NOS | 1185 (19.6%) | 460 (16.5%) | 613 (12.8%) | 2258 (16.6%) | < .01 |
| Cerebrum | 4490 (74.2%) | 2270 (81.2%) | 4126 (86.4%) | 10886 (79.9%) | ||
| Midline | 375 (6.2%) | 65 (2.3%) | 37 (0.8%) | 477 (3.5%) | ||
| Charlson- | 0 | 5134 (84.9%) | 2441 (87.3%) | 4084 (85.5%) | 11659 (85.6%) | 0.07 |
| Deyo | 1 | 653 (10.8%) | 251 (9.0%) | 500 (10.5%) | 1404 (10.3%) | |
| Score | 2 | 204 (3.4%) | 73 (2.6%) | 149 (3.1%) | 426 (3.1%) | |
| ≥3 | 59 (1.0%) | 30 (1.1%) | 43 (0.9%) | 132 (1.0%) | ||
| Facility | Unknown | 2720 (45.0%) | 1458 (52.2%) | 2134 (44.7%) | 6312 (46.3%) | < .01 |
| Type | Community | 1660 (27.4%) | 568 (20.3%) | 1130 (23.7%) | 3358 (24.7%) | |
| Academic | 1670 (27.6%) | 769 (27.5%) | 1512 (31.7%) | 3951 (29.0%) | ||
| Living area | Unknown | 247 (4.1%) | 114 (4.1%) | 142 (3.0%) | 503 (3.7%) | 0.96 |
| Metro | 4834 (79.9%) | 2237 (80.0%) | 3870 (81.0%) | 10941 (80.3%) | ||
| Rural | 969 (16.0%) | 444 (15.9%) | 764 (16.0%) | 2177 (16.0%) | ||
| Treatment | Unknown | 334 (5.5%) | 146 (5.2%) | 277 (5.8%) | 757 (5.6%) | < .01 |
| None | 629 (10.4%) | 205 (7.3%) | 275 (5.8%) | 1109 (8.1%) | ||
| RT+CT+S | 692 (11.4%) | 348 (12.5%) | 415 (8.7%) | 1455 (10.7%) | ||
| RT+CT | 588 (9.7%) | 167 (6.0%) | 159 (3.3%) | 914 (6.7%) | ||
| RT+S | 736 (12.2%) | 377 (13.5%) | 456 (9.5%) | 1569 (11.5%) | ||
| CT+S | 167 (2.8%) | 168 (6.0%) | 561 (11.7%) | 896 (6.6%) | ||
| S only | 2202 (36.4%) | 1182 (42.3%) | 2274 (47.6%) | 5658 (41.5%) | ||
| RT only | 565 (9.3%) | 124 (4.4%) | 145 (3.0%) | 834 (6.1%) | ||
| CT only | 137 (2.3%) | 78 (2.8%) | 214 (4.5%) | 429 (3.1%) | ||
| Insurance status | Not Insured | 412 (6.8%) | 209 (7.5%) | 326 (6.8%) | 947 (7.0%) | < .01 |
| Private | 3859 (63.8%) | 1896 (67.8%) | 3390 (71.0%) | 9145 (67.1%) | ||
| Medicaid | 661 (10.9%) | 329 (11.8%) | 479 (10.0%) | 1469 (10.8%) | ||
| Medicare | 880 (14.5%) | 255 (9.1%) | 398 (8.3%) | 1533 (11.3%) | ||
| Other Gov’t | 112 (1.9%) | 55 (2.0%) | 87 (1.8%) | 254 (1.9%) | ||
| Unknown | 126 (2.1%) | 51 (1.8%) | 96 (2.0%) | 273 (2.0%) |
NOS: Not otherwise specified; RT: Radiation therapy; CT: Chemotherapy; S: Surgery
Table 3. Characteristics of the study population, by 1p/19q co-deletion status.
| 1p/19q co-deleted N = 901 |
Non co-deleted N = 1003 |
Incomplete N = 196 |
Unknown N = 11521 |
All N = 13621 |
p-value | ||
|---|---|---|---|---|---|---|---|
| Age | Median(years) | 37 (18–83) | 42 (18–80) | 38 (18–79) | 41 (18–90) | 41 (18–90) | < .01 |
| Gender | Male | 511 (56.7%) | 562 (56.0%) | 110 (56.1%) | 6447(56.0%) | 7630(56.0%) | 0.98 |
| Female | 390 (43.3%) | 441 (44.0%) | 86 (43.9%) | 5074(44.0%) | 5991(44.0%) | ||
| Race | White | 798 (88.6%) | 902 (89.9%) | 172 (87.8%) | 10179(88.4%) | 12051(88.5%) | 0.02 |
| Black | 51 (5.7%) | 46 (4.6%) | 11 (5.6%) | 729 (6.3%) | 837 (6.1%) | ||
| Other | 43 (4.8%) | 50 (5.0%) | 9 (4.6%) | 431 (3.7%) | 533 (3.9%) | ||
| Unknown | 9 (1.0%) | 5 (0.5%) | 4 (2.0%) | 182 (1.6%) | 200 (1.5%) | ||
| Hispanic | Unknown | 23 (2.6%) | 29 (2.9%) | 7 (3.6%) | 809 (7.0%) | 868 (6.4%) | <0.01 |
| No | 810 (89.9%) | 907 (90.4%) | 174 (88.8%) | 9840 (85.4%) | 11731(86.1%) | ||
| Yes | 68 (7.5%) | 67 (6.7%) | 15 (7.7%) | 872 (7.6%) | 1022 (7.5%) | ||
| Tumor location | NOS | 118 (13.1%) | 116 (11.6%) | 35 (17.9%) | 1989 (17.3%) | 2258 (16.6%) | < .01 |
| Cerebrum | 764 (84.8%) | 884 (88.1%) | 159 (81.1%) | 9079 (78.8%) | 10886(79.9%) | ||
| Midline | 19 (2.1%) | 3 (0.3%) | 2 (1.0%) | 453 (3.9%) | 477 (3.5%) | ||
| Charlson- | 0 | 774 (85.9%) | 850 (84.7%) | 168 (85.7%) | 9867 (85.6%) | 11659(85.6%) | 0.76 |
| Deyo | 1 | 93 (10.3%) | 101 (10.1%) | 20 (10.2%) | 1190 (10.3%) | 1404 (10.3%) | |
| Score | 2 | 23 (2.6%) | 38 (3.8%) | 7 (3.6%) | 358 (3.1%) | 426 (3.1%) | |
| ≥3 | 11 (1.2%) | 14 (1.4%) | 1 (0.5%) | 106 (0.9%) | 132 (1.0%) | ||
| Facility | Unknown | 518 (57.5%) | 443 (44.2%) | 105 (53.6%) | 5246 (45.5%) | 6312 (46.3%) | < .01 |
| Type | Community | 135 (15.0%) | 225 (22.4%) | 38 (19.4%) | 2960 (25.7%) | 3358 (24.7%) | |
| Academic | 248 (27.5%) | 335 (33.4%) | 53 (27.0%) | 3315 (28.8%) | 3951 (29.0%) | ||
| Living area | Unknown | 26 (2.9%) | 34 (3.4%) | 4 (2.0%) | 439 (3.8%) | 503 (3.7%) | 0.96 |
| Metro | 730 (81.0%) | 797 (79.5%) | 152 (77.6%) | 9262 (80.4%) | 10941(80.3%) | ||
| Rural | 145 (16.1%) | 172 (17.1%) | 40 (20.4%) | 1820 (15.8%) | 2177 (16.0%) | ||
| Treatment | Unknown | 37 (4.1%) | 48 (4.8%) | 7 (3.6%) | 665 (5.8%) | 757 (5.6%) | < .01 |
| None | 40 (4.4%) | 40 (4.0%) | 7 (3.6%) | 1022 (8.9%) | 1109 (8.1%) | ||
| RT+CT+S | 138 (15.3%) | 102 (10.2%) | 31 (15.8%) | 1184 (10.3%) | 1455 (10.7%) | ||
| RT+CT | 41 (4.6%) | 36 (3.6%) | 12 (6.1%) | 825 (7.2%) | 914 (6.7%) | ||
| RT+S | 115 (12.8%) | 79 (7.9%) | 27 (13.8%) | 1348 (11.7%) | 1569 (11.5%) | ||
| CT+S | 56 (6.2%) | 181 (18.0%) | 14 (7.1%) | 645 (5.6%) | 896 (6.6%) | ||
| S only | 417 (46.3%) | 444 (44.3%) | 92 (46.9%) | 4705 (40.8%) | 5658 (41.5%) | ||
| RT only | 39 (4.3%) | 21 (2.1%) | 2 (1.0%) | 772 (6.7%) | 834 (6.1%) | ||
| CT only | 18 (2.0%) | 52 (5.2%) | 4 (2.0%) | 355 (3.1%) | 429 (3.1%) | ||
| Insurance status | Not Insured | 57 (6.3%) | 68 (6.8%) | 14 (7.1%) | 808 (7.0%) | 947 (7.0%) | < .01 |
| Private | 639 (70.9%) | 741 (73.9%) | 136 (69.4%) | 7629 (66.2%) | 9145 (67.1%) | ||
| Medicaid | 110 (12.2%) | 98 (9.8%) | 25 (12.8%) | 1236 (10.7%) | 1469 (10.8%) | ||
| Medicare | 69 (7.7%) | 72 (7.2%) | 18 (9.2%) | 1374 (11.9%) | 1533 (11.3%) | ||
| Other Gov’t | 20 (2.2%) | 18 (1.8%) | 1 (0.5%) | 215 (1.9%) | 254 (1.9%) | ||
| Unknown | 6 (0.7%) | 6 (0.6%) | 2 (1.0%) | 259 (2.2%) | 273 (2.0%) |
NOS: Not otherwise specified; RT: Radiation therapy; CT: Chemotherapy; S: Surgery
Astrocytic histology included 6,050 (44.4%) patients, oligodendroglial included 4,776 (35.1%), and mixed comprised 2,795 (20.5%) patients (Table 2). Gender distribution and Hispanic origin were not different among histology groups. Black race was more common among astrocytic tumors (7.2%). The most common primary site location was the cerebrum (79.9%), followed by “other” (16.6%), and midline (3.5%). 1p/19q co-deletion status was unknown in 9,617 patients (84.6%), reported as co-deleted in 901 (6.6%), non-co-deleted in 1,003 (7.4%), and incomplete in 196 (1.4%) patients (Table 3). 1p/19q co-deletion testing is only available after 2010 with an increasing tendency. Patients with 1p/19q co-deletion were significantly younger than the non-co-deleted or incomplete groups, with a median age of 37 years (p<0.01). Gender and Hispanic distribution were similar among all groups (Table 1, Table 2) in a comparison of molecular factors (p = 0.98). A higher frequency of patients with 1p/19q co-deletion had a midline primary site location compared to those with 1p/19q non-co-deleted (2.1% versus 0.3%, p<0.01).
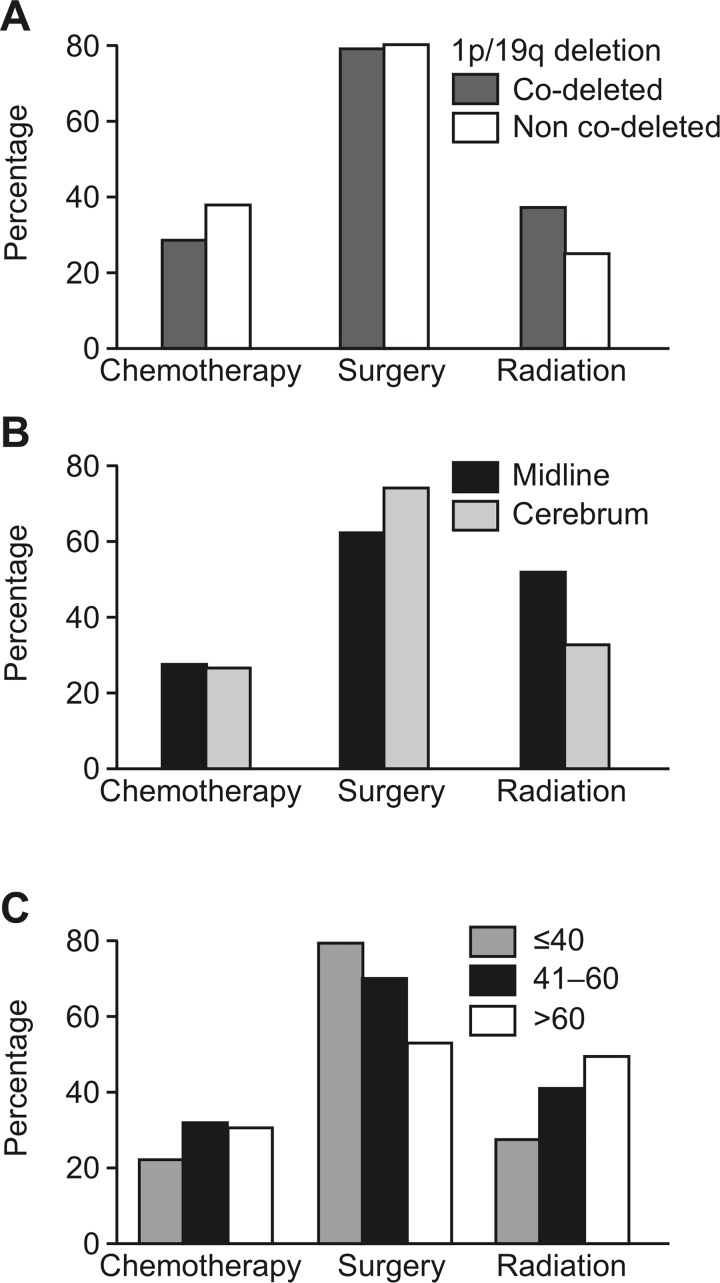
Overall, the most common treatment modality was surgery (72.2%), followed by radiation (36.0%), and chemotherapy (27.3%); 41.5% were treated with surgery only (Fig 1). Chemotherapy and radiation were generally administered as adjuvant treatment; 6.1% of the patients received radiation only and 3.1% received chemotherapy only. The maximal treatment combination (radiation, chemotherapy, and surgery) was used in 10.7% of the patients. Other combinations included radiation and surgery (11.5%), chemotherapy and surgery (6.6%), and radiotherapy and chemotherapy (6.7%). Treatment modality was unknown in 5.6% of the cases, and 8.1% received no treatment.
Fig 1.
A. Chemotherapy, surgery, and radiation treatment receipt by 1p/19q co-deletion status. B. Chemotherapy, surgery, and radiation treatment receipt by tumor location. C. Chemotherapy, surgery, and radiation treatment receipt by age.
Treatment varied significantly with age (Table 1, Fig 1). The percent of patients undergoing gross total resection decreased with increasing age. The use of adjuvant treatment was more common in patients older than 40 years of age. Radiation only, radiation plus chemotherapy, and no treatment were more common in patients over 60 years of age. Primary site location was also a main determinant of treatment. Patients with midline lesions were less likely to be treated with surgery alone and more commonly received radiation compared to primary site location in the cerebrum. Interestingly, there was a higher percentage of astrocytic tumors with midline primary site location (6.2%), compared to oligodendroglial tumors (0.8%). Oligodendroglial tumors were also more likely to be located in the cerebrum compared to astrocytic tumors.
Histological classification and molecular signatures had an impact on treatment receipt (Table 2, Fig 1). Astrocytic tumors were more likely to be treated with radiation (43.8% versus 25.3%, p<0.01) while oligodendroglial tumors were more likely to receive surgery (79.9% versus 64.6%, p<0.01). Chemotherapy did not vary between both groups (p = 0.07). Adjuvant treatment was more common in patients with astrocytic histology. Patients with oligodendroglial histology were more likely to be treated with surgery only or chemotherapy plus surgery, and were likely to receive radiation.
Radiation was more frequent in patients with 1p/19q co-deleted than in patients with non-co-deleted status (37.3% versus 24.3%, p<0.01) (Table 3, Fig 1). Chemotherapy was more common in patients with 1p/19q non-co-deleted than in those with 1p/19q co-deleted (37.4% versus 28.1%, p<0.01). Combination therapy with radiation, chemotherapy plus surgery, radiation plus chemotherapy, and radiation plus surgery were more common in patients with 1p/19q co-deleted (15.3%, 4.6%, and 12.8%, respectively). Combined chemotherapy and surgery was more frequent in patients with 1p/19q non-co-deleted (18%).
Socioeconomic factors were assessed by insurance status, income level, and dwelling area. The most common primary payer was private insurance (67.2%), followed by Medicare (11.9%), and Medicaid (10.8%). Government insurance covered 23.9% of patients. The type of primary payer varied with age and histologic groups. The majority of patients older than 60 years of age were covered by a type of government insurance (65.6%); primarily by Medicare (61.8%). Private insurance was the main coverage in patients younger than 60 years while 17.3% were covered by any type of government insurance.
Median annual income was higher than $38,000 per year in 83.7% of patients. Socioeconomic status was somewhat lower in patients with astrocytic tumors with lower income and educational variables. Over 80% of the patients lived in metropolitan areas. The proportion of patients living in rural areas was significantly higher for patients older than 60 years of age. Histology groups were not associated with area of residence. Twenty-nine percent of patients were reported to have received treatment in academic facilities, while 25% were treated in community hospitals. The setting of the facility were patients were treated was unknown in 46.3%.
Survival
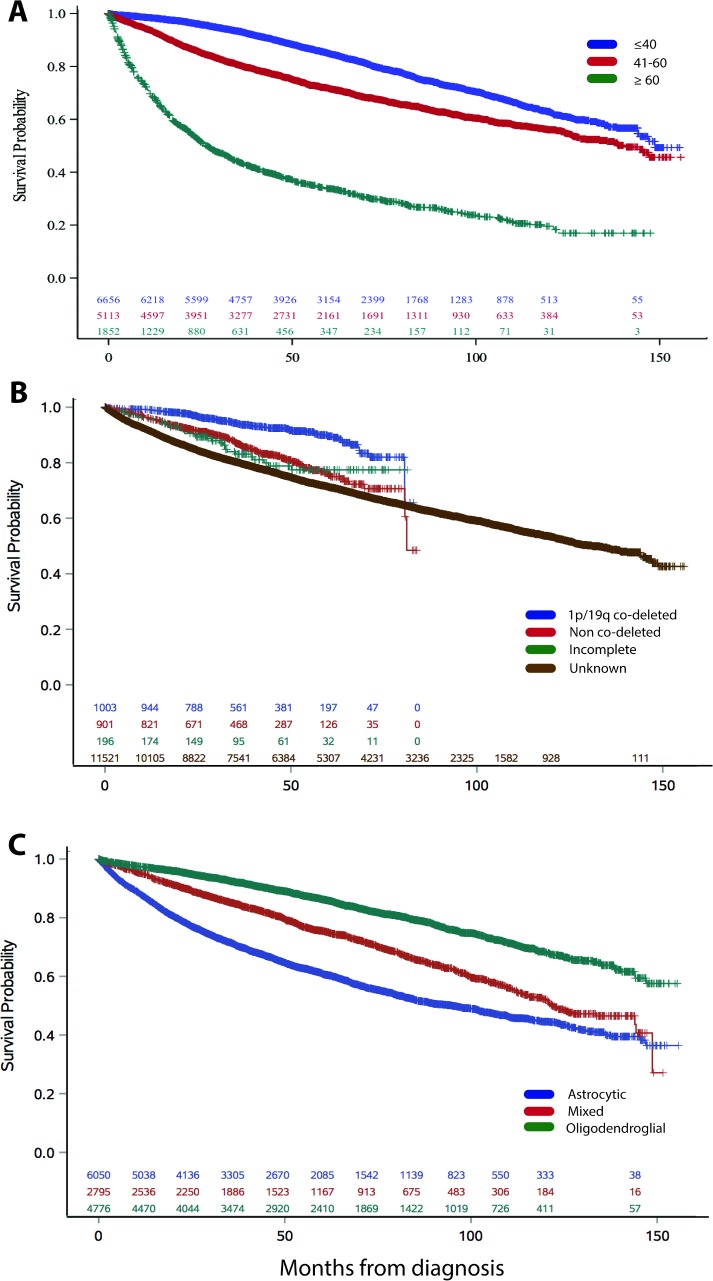
Median overall survival was 11.3 years. Survival varied by age, comorbidities, tumor location, histology group, and molecular predictors; younger age, fewer comorbidities, oligodendroglial histology, 1p/19q co-deletion, and cerebrum location had the best prognosis (Fig 2, Fig 3). Median survival was greater in patients younger than 60 years of age (12.4 and 11.7 years for <40 and 41–60 years old), compared to 2.2 years in patients older than 60 years. Median survival was not reached in patients with 1p/19q co-deleted low-grade gliomas. Median survival was 7.9 years in astrocytic tumors, 10 years in mixed histology, and was not reached in oligodendroglial tumors.
Fig 2.
A. Survival by age group. B. Survival by 1p/19q co-deletion status. C. Survival by histology group.
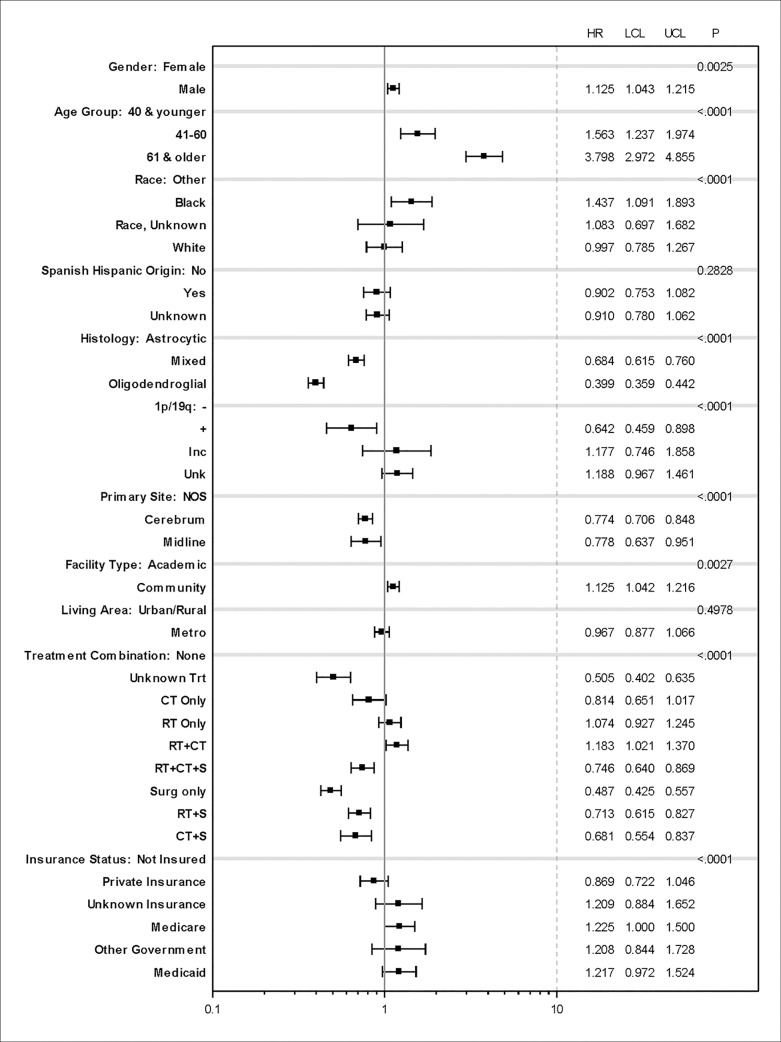
Fig 3. Multivariate analysis for risk factor for mortality.
We performed a multivariate analysis for risk factors for mortality (Fig 3). No treatment, radiation only, and chemotherapy plus radiation were associated with increased risk of mortality. Male gender and black race, as compared to other, were associated with mortality. Medicaid and Medicare, and treatment in community facilities were associated with an increased risk of death as compared to not insured.
Discussion
With the updated WHO of the nervous system in 2016 molecular profiling is required for proper low-grade glioma classification[1]. Risk assessment is based on three groups: IDH mutant tumors with 1p/19q co-deletion (predominantly oligodendroglial), IDH mutant without 1p/19q co-deletion (predominantly astrocytic), and IDH wild-type tumors[19–21]. The NCDB participant user file from 2004 to 2015 uses the WHO 2007 classification and some molecular markers such as IDH status were not available in our study[16, 22]. Molecular profiling reports were available after 2010 for 15.41% of the patients (Table 3), with an increasing testing tendency.
In NCDB, overall survival was 11 years for all patients evaluated in CoC accredited hospitals in the United States. This is comparable to the 13-year median survival reported by Buckner et al. for high-risk patients receiving chemotherapy and radiation[23], and surpasses the median survival reported in the majority of clinical trials, where it ranges from 7 to 9 years[24, 25]. Age 60 years and younger, 1p19q co-deletion, and oligodendroglial tumors were factors associated with best survival in Kaplan-Meier analyses (Fig 2), and the results held in multivariate proportional hazards models (Fig 3). Tumor location predicts the aggressiveness of treatment due to the risk of functional impairment, that affects long-term survival[26]. Location, such as midline location is also associated with molecular profiling characteristics associated with poor prognosis[8, 27, 28].
Sociodemographic factors that include income, type of insurance, and facility location (academic versus community) are also associated with mortality. Patients treated in community setting had an increased risk of mortality compared to patients treated in academic centers. Similar findings have been described in glioblastoma[29], and suggests a role in access to neuro-oncological care and the role of hospital volume and physician’s expertise in treatment decisions. However, differences in risk factor exposure, disease severity, and population differences may also account for the observed results.
High-risk features for mortality in patients with diagnosis of low-grade gliomas include age older than 40 years, tumor diameter greater than 6 cm, midline crossing, presence of neurological deficit, and astrocytic histology[10]. Shaw et al. determined that patients defined as low risk after gross total resection have a 50% risk of tumor progression at 5 years[11]. Based on this, active surveillance remains an option for low risk patients[11]. However, due to the overlapping molecular prognostic factors, heterogeneity of these tumors, and challenges of completing clinical trials in a rarer and long surviving cancer, treatment recommendations remain unestablished.
The longevity associated with these tumors provides concerns for long-term treatment-associated toxicities. Treatment for brain tumors carries the concern for cognitive and functional impairment[30], yet, not receiving treatment allowing for disease progression with associated neurologic deficits and significant mortality.
Our results reflect that patients who receive chemotherapy plus surgery reach comparable survival to those receiving surgery alone. Iwadate et al. found similar results in a nonrandomized trial[31], reporting equivalent outcomes in patients who underwent surgery alone versus those who received adjuvant chemotherapy. The benefits of surgery with maximal safe resection are established[32]. Our study with over 70% receiving surgery as first course of treatment demonstrates the benefit of this and is in keeping with the literature, as surgical resection and the extent of the resection has a significant survival benefit[33–35].
The recommendation of chemotherapy as adjuvant treatment is based on the extent of resection, molecular features, and general state of patient. Chemotherapy is an option in patients with a favorable molecular profile, and as a way to delay or forgo radiation[36]. Acknowledging gliomas as a wider brain involved disease than based on imaging, and that the majority of tumors will progress without further treatment[37], the use of chemotherapy is an option for selected high-risk patients to delay disease progression.
Triple combination therapy of surgery plus chemotherapy plus radiation was uncommon (10.7%) but was associated with survival benefits. Retrospective analysis have shown similar results[38]. Clinical trials have demonstrated that combination procarbazine, lomustine and vincristine plus radiation improves outcomes in high risk patients[23, 25]. The type of chemotherapy agent received is not detailed in NCDB and is a limitation to NCDB analysis. Procarbazine, lomustine and vincristine (PCV) combination or temozolomide are the most used regimens[39], and are thought to be comparable. Adjuvant temozolomide have demonstrated survival benefits in anaplastic gliomas and high-risk low grade gliomas[39, 40].
Chemotherapy alone did not demonstrate survival benefits when compared to radiation alone, or radiation plus chemotherapy in a phase III randomized clinical trials[41, 42], but was associated with survival benefits in our analysis. Single center experiences and retrospective analyses in unresectable tumors have shown some benefits[43, 44]. Chemotherapy alone may be of benefit in selected patients in whom resection is not feasible.
The use of radiation alone and radiation plus chemotherapy was associated with increased risk of mortality. Radiation use was significantly higher in patients with 1p/19q co-deleted, older age, and astrocytic histology. These patients may have had less proper tumor resective surgery due to their age and disease burden, and/or a more aggressive clinical presentation. As to why 1p/19q co-deletion are more associated with radiation, it is possibly due to late disease presentation, or being more aggressive on a treatment responsive cancer. Although analyzing different clinical trials are problematic, the upfront radiation alone trials without chemotherapy have median survival rates that are less than a decade[39, 45, 46]. Early radiation has not shown to improve overall survival in randomized trials compared to patients who receive radiation at the time of progression[45]. The use of radiation is associated with significant adverse events, mainly neurocognitive deterioration, vasculopathies, and secondary malignancies[30, 47], and remains a topic of discussion as first line treatment.
NCDB provides long-term follow up and quality data on first course of treatment, but does not include treatment associated complications, or cause of death. Molecular profiling and tumor characteristics beyond our study are not widely available in the data, as well as individual patient assessments. However, our investigation provides an unique opportunity to evaluate patterns of care across the largest cancer registry[15].
In summary, outcomes for low-grade gliomas in most of United States is at or beyond reported in clinical trials and the use of adjuvant therapy may be associated with survival benefits in selected high-risk patients. The role of radiation remains under investigation as first course of treatment in all low-grade gliomas. Outcomes will continue to improve with further understanding of tumor biology and behavior, improving patient selection.
Supporting information
(TIF)
Data Availability
The data is provided by the National Cancer Database and is available upon request by the National Cancer Database for investigators associated with Commission on Cancer accredited institutions. The National Cancer Database retains the decision of accessing the data based on an application process. Association to a Commission on Cancer accredited cancer program is required for applying. The application process opens throughout the year and requires a protocol submission. We applied for and were granted access to the data in the fall of 2017. Details on obtaining participant user files for data analysis is available in the following URL: https://na01.safelinks.protection.outlook.com/?url=https%3A%2F%2Fwww.facs.org%2Fquality-programs%2Fcancer%2Fncdb%2Fpuf&data=02%7C01%7Cjlvillano%40uky.edu%7C3e1f308e33a24f5e821608d60f427ddf%7C2b30530b69b64457b818481cb53d42ae%7C0%7C0%7C636713175589924852&sdata=siZFFXbVtzNa5qO4xWijysSJw3y%2FlA8uORwVyCub0C8%3D&reserved=0. Finally, collaborative interactions is an option for researchers outside of the Commission on Cancer accredited institutions, but data management and analysis (no data transfer) must be performed by the PI of the protocol approved from a Commission on Cancer accredited institution.
Funding Statement
Ms. Slone was supported by the Biostatistics and Bioinformatics Shared Resource of the University of Kentucky Markey Cancer Center (P30CA177558).
References
- 1.Cavenee WK, Louis DN, Ohgaki H, Wiestler OD, International Agency for Research on Cancer WHO classification of tumours of the central nervous system. Revised 4th edition ed. Lyon: International Agency For Research On Cancer; 2016. 408 pages p. [Google Scholar]
- 2.Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta neuropathologica. 2016;131(6):803–20. Epub 2016/05/10. 10.1007/s00401-016-1545-1 . [DOI] [PubMed] [Google Scholar]
- 3.Ostrom QT, Gittleman H, Xu J, Kromer C, Wolinsky Y, Kruchko C, et al. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2009–2013. Neuro-oncology. 2016;18(suppl_5):v1–v75. Epub 2017/05/06. 10.1093/neuonc/now207 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jakola AS, Myrmel KS, Kloster R, Torp SH, Lindal S, Unsgard G, et al. Comparison of a strategy favoring early surgical resection vs a strategy favoring watchful waiting in low-grade gliomas. Jama. 2012;308(18):1881–8. Epub 2012/10/27. 10.1001/jama.2012.12807 . [DOI] [PubMed] [Google Scholar]
- 5.Aghi MK, Nahed BV, Sloan AE, Ryken TC, Kalkanis SN, Olson JJ. The role of surgery in the management of patients with diffuse low grade glioma: A systematic review and evidence-based clinical practice guideline. Journal of neuro-oncology. 2015;125(3):503–30. Epub 2015/11/05. 10.1007/s11060-015-1867-1 . [DOI] [PubMed] [Google Scholar]
- 6.Chang EF, Potts MB, Keles GE, Lamborn KR, Chang SM, Barbaro NM, et al. Seizure characteristics and control following resection in 332 patients with low-grade gliomas. Journal of neurosurgery. 2008;108(2):227–35. Epub 2008/02/05. 10.3171/JNS/2008/108/2/0227 . [DOI] [PubMed] [Google Scholar]
- 7.Chen H, Judkins J, Thomas C, Wu M, Khoury L, Benjamin CG, et al. Mutant IDH1 and seizures in patients with glioma. Neurology. 2017;88(19):1805–13. Epub 2017/04/14. 10.1212/WNL.0000000000003911 ; PubMed Central PMCID: PMCPMC5419985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brat DJ, Verhaak RG, Aldape KD, Yung WK, Salama SR, Cooper LA, et al. Comprehensive, Integrative Genomic Analysis of Diffuse Lower-Grade Gliomas. The New England journal of medicine. 2015;372(26):2481–98. Epub 2015/06/11. 10.1056/NEJMoa1402121 ; PubMed Central PMCID: PMCPmc4530011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eckel-Passow JE, Lachance DH, Molinaro AM, Walsh KM, Decker PA, Sicotte H, et al. Glioma Groups Based on 1p/19q, IDH, and TERT Promoter Mutations in Tumors. The New England journal of medicine. 2015;372(26):2499–508. Epub 2015/06/11. 10.1056/NEJMoa1407279 ; PubMed Central PMCID: PMCPmc4489704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pignatti F, van den Bent M, Curran D, Debruyne C, Sylvester R, Therasse P, et al. Prognostic factors for survival in adult patients with cerebral low-grade glioma. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2002;20(8):2076–84. Epub 2002/04/17. 10.1200/jco.2002.08.121 . [DOI] [PubMed] [Google Scholar]
- 11.Shaw EG, Berkey B, Coons SW, Bullard D, Brachman D, Buckner JC, et al. Recurrence following neurosurgeon-determined gross-total resection of adult supratentorial low-grade glioma: results of a prospective clinical trial. Journal of neurosurgery. 2008;109(5):835–41. Epub 2008/11/04. 10.3171/JNS/2008/109/11/0835 ; PubMed Central PMCID: PMCPMC3833272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ziu M, Kalkanis SN, Gilbert M, Ryken TC, Olson JJ. The role of initial chemotherapy for the treatment of adults with diffuse low grade glioma: A systematic review and evidence-based clinical practice guideline. Journal of neuro-oncology. 2015;125(3):585–607. Epub 2015/11/05. 10.1007/s11060-015-1931-x . [DOI] [PubMed] [Google Scholar]
- 13.Chao ST, Suh JH. When should radiotherapy for low-grade glioma be given—immediately after surgery or at the time of progression? Nature clinical practice Oncology. 2006;3(3):136–7. Epub 2006/03/08. 10.1038/ncponc0455 . [DOI] [PubMed] [Google Scholar]
- 14.Surawicz TS, Davis F, Freels S, Laws ER Jr., Menck HR. Brain tumor survival: results from the National Cancer Data Base. Journal of neuro-oncology. 1998;40(2):151–60. Epub 1999/01/19. . [DOI] [PubMed] [Google Scholar]
- 15.Boffa DJ, Rosen JE, Mallin K, Loomis A, Gay G, Palis B, et al. Using the National Cancer Database for Outcomes Research: A Review. JAMA oncology. 2017;3(12):1722–8. Epub 2017/02/28. 10.1001/jamaoncol.2016.6905 . [DOI] [PubMed] [Google Scholar]
- 16.American College of Surgeons. National Cancer Database. https://www.facs.org/quality-programs/cancer/ncdb. Accessed January 23, 2018. [cited 2018 1/23]. Available from: https://www.facs.org/quality-programs/cancer/ncdb.
- 17.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of chronic diseases. 1987;40(5):373–83. Epub 1987/01/01. . [DOI] [PubMed] [Google Scholar]
- 18.Liu M, Thakkar JP, Garcia CR, Dolecek TA, Wagner LM, Dressler EVM, et al. National cancer database analysis of outcomes in pediatric glioblastoma. Cancer medicine. 2018. Epub 2018/03/14. 10.1002/cam4.1404 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Killela PJ, Pirozzi CJ, Healy P, Reitman ZJ, Lipp E, Rasheed BA, et al. Mutations in IDH1, IDH2, and in the TERT promoter define clinically distinct subgroups of adult malignant gliomas. Oncotarget. 2014;5(6):1515–25. Epub 2014/04/12. doi: 10.18632/oncotarget.1765 ; PubMed Central PMCID: PMCPMC4039228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiao Y, Killela PJ, Reitman ZJ, Rasheed AB, Heaphy CM, de Wilde RF, et al. Frequent ATRX, CIC, FUBP1 and IDH1 mutations refine the classification of malignant gliomas. Oncotarget. 2012;3(7):709–22. Epub 2012/08/08. doi: 10.18632/oncotarget.588 ; PubMed Central PMCID: PMCPMC3443254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wiestler B, Capper D, Holland-Letz T, Korshunov A, von Deimling A, Pfister SM, et al. ATRX loss refines the classification of anaplastic gliomas and identifies a subgroup of IDH mutant astrocytic tumors with better prognosis. Acta neuropathologica. 2013;126(3):443–51. Epub 2013/08/02. 10.1007/s00401-013-1156-z . [DOI] [PubMed] [Google Scholar]
- 22.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta neuropathologica. 2007;114(2):97–109. 10.1007/s00401-007-0243-4 ; PubMed Central PMCID: PMCPMC1929165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buckner JC, Shaw EG, Pugh SL, Chakravarti A, Gilbert MR, Barger GR, et al. Radiation plus Procarbazine, CCNU, and Vincristine in Low-Grade Glioma. The New England journal of medicine. 2016;374(14):1344–55. Epub 2016/04/07. 10.1056/NEJMoa1500925 ; PubMed Central PMCID: PMCPMC5170873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wahl M, Phillips JJ, Molinaro AM, Lin Y, Perry A, Haas-Kogan DA, et al. Chemotherapy for adult low-grade gliomas: clinical outcomes by molecular subtype in a phase II study of adjuvant temozolomide. Neuro-oncology. 2017;19(2):242–51. Epub 2016/08/31. 10.1093/neuonc/now176 ; PubMed Central PMCID: PMCPMC5464133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shaw EG, Wang M, Coons SW, Brachman DG, Buckner JC, Stelzer KJ, et al. Randomized trial of radiation therapy plus procarbazine, lomustine, and vincristine chemotherapy for supratentorial adult low-grade glioma: initial results of RTOG 9802. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2012;30(25):3065–70. Epub 2012/08/02. 10.1200/jco.2011.35.8598 ; PubMed Central PMCID: PMCPMC3732006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang EF, Clark A, Smith JS, Polley MY, Chang SM, Barbaro NM, et al. Functional mapping-guided resection of low-grade gliomas in eloquent areas of the brain: improvement of long-term survival. Clinical article. Journal of neurosurgery. 2011;114(3):566–73. Epub 2010/07/20. 10.3171/2010.6.JNS091246 ; PubMed Central PMCID: PMCPMC3877621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verhaak RGW, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, et al. An integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR and NF1. Cancer cell. 2010;17(1):98 10.1016/j.ccr.2009.12.020 ; PubMed Central PMCID: PMCPMC2818769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guan X, Vengoechea J, Zheng S, Sloan AE, Chen Y, Brat DJ, et al. Molecular subtypes of glioblastoma are relevant to lower grade glioma. PloS one. 2014;9(3):e91216 Epub 2014/03/13. 10.1371/journal.pone.0091216 ; PubMed Central PMCID: PMCPMC3948818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koshy M, Sher DJ, Spiotto M, Husain Z, Engelhard H, Slavin K, et al. Association between hospital volume and receipt of treatment and survival in patients with glioblastoma. Journal of neuro-oncology. 2017;135(3):529–34. Epub 2017/08/25. 10.1007/s11060-017-2598-2 . [DOI] [PubMed] [Google Scholar]
- 30.Klein M. Neurocognitive functioning in adult WHO grade II gliomas: impact of old and new treatment modalities. Neuro-oncology. 2012;14(suppl_4):iv17–iv24. 10.1093/neuonc/nos161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iwadate Y, Matsutani T, Hasegawa Y, Shinozaki N, Higuchi Y, Saeki N. Favorable long-term outcome of low-grade oligodendrogliomas irrespective of 1p/19q status when treated without radiotherapy. Journal of neuro-oncology. 2011;102(3):443–9. Epub 2010/08/20. 10.1007/s11060-010-0340-4 . [DOI] [PubMed] [Google Scholar]
- 32.Youland RS, Brown PD, Giannini C, Parney IF, Uhm JH, Laack NN. Adult low-grade glioma: 19-year experience at a single institution. American journal of clinical oncology. 2013;36(6):612–9. Epub 2012/08/16. 10.1097/COC.0b013e31825d580a ; PubMed Central PMCID: PMCPMC4361933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kilic T, Ozduman K, Elmaci I, Sav A, Necmettin Pamir M. Effect of surgery on tumor progression and malignant degeneration in hemispheric diffuse low-grade astrocytomas. Journal of clinical neuroscience: official journal of the Neurosurgical Society of Australasia. 2002;9(5):549–52. Epub 2002/10/18. . [DOI] [PubMed] [Google Scholar]
- 34.Smith JS, Chang EF, Lamborn KR, Chang SM, Prados MD, Cha S, et al. Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2008;26(8):1338–45. Epub 2008/03/08. 10.1200/jco.2007.13.9337 . [DOI] [PubMed] [Google Scholar]
- 35.McGirt MJ, Chaichana KL, Gathinji M, Attenello FJ, Than K, Olivi A, et al. Independent association of extent of resection with survival in patients with malignant brain astrocytoma. Journal of neurosurgery. 2009;110(1):156–62. Epub 2008/10/14. 10.3171/2008.4.17536 . [DOI] [PubMed] [Google Scholar]
- 36.Higuchi Y, Iwadate Y, Yamaura A. Treatment of low-grade oligodendroglial tumors without radiotherapy. Neurology. 2004;63(12):2384–6. Epub 2004/12/30. . [DOI] [PubMed] [Google Scholar]
- 37.Ricard D, Kaloshi G, Amiel-Benouaich A, Lejeune J, Marie Y, Mandonnet E, et al. Dynamic history of low-grade gliomas before and after temozolomide treatment. Annals of neurology. 2007;61(5):484–90. Epub 2007/05/01. 10.1002/ana.21125 . [DOI] [PubMed] [Google Scholar]
- 38.Nakamura M, Konishi N, Tsunoda S, Nakase H, Tsuzuki T, Aoki H, et al. Analysis of prognostic and survival factors related to treatment of low-grade astrocytomas in adults. Oncology. 2000;58(2):108–16. Epub 2000/03/08. 10.1159/000012087 . [DOI] [PubMed] [Google Scholar]
- 39.Fisher BJ, Hu C, Macdonald DR, Lesser GJ, Coons SW, Brachman DG, et al. Phase 2 study of temozolomide-based chemoradiation therapy for high-risk low-grade gliomas: preliminary results of Radiation Therapy Oncology Group 0424. International journal of radiation oncology, biology, physics. 2015;91(3):497–504. Epub 2015/02/15. 10.1016/j.ijrobp.2014.11.012 ; PubMed Central PMCID: PMCPMC4329190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van den Bent MJ, Baumert B, Erridge SC, Vogelbaum MA, Nowak AK, Sanson M, et al. Interim results from the CATNON trial (EORTC study 26053–22054) of treatment with concurrent and adjuvant temozolomide for 1p/19q non-co-deleted anaplastic glioma: a phase 3, randomised, open-label intergroup study. Lancet (London, England). 2017;390(10103):1645–53. Epub 2017/08/13. 10.1016/s0140-6736(17)31442-3 ; PubMed Central PMCID: PMCPMC5806535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jaeckle K, Vogelbaum M, Ballman K, Anderson SK, Giannini C, Aldape K, et al. CODEL (Alliance-N0577; EORTC-26081/22086; NRG-1071; NCIC-CEC-2): Phase III Randomized Study of RT vs. RT+TMZ vs. TMZ for Newly Diagnosed 1p/19q-Codeleted Anaplastic Oligodendroglial Tumors. Analysis of Patients Treated on the Original Protocol Design (PL02.005). Neurology. 2016;86(16 Supplement). [Google Scholar]
- 42.Baumert BG, Hegi ME, van den Bent MJ, von Deimling A, Gorlia T, Hoang-Xuan K, et al. Temozolomide chemotherapy versus radiotherapy in high-risk low-grade glioma (EORTC 22033–26033): a randomised, open-label, phase 3 intergroup study. The Lancet Oncology. 2016;17(11):1521–32. Epub 2016/10/01. 10.1016/S1470-2045(16)30313-8 ; PubMed Central PMCID: PMCPMC5124485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frenay MP, Fontaine D, Vandenbos F, Lebrun C. First-line nitrosourea-based chemotherapy in symptomatic non-resectable supratentorial pure low-grade astrocytomas. European journal of neurology. 2005;12(9):685–90. Epub 2005/09/01. 10.1111/j.1468-1331.2005.01028.x . [DOI] [PubMed] [Google Scholar]
- 44.Stege EM, Kros JM, de Bruin HG, Enting RH, van Heuvel I, Looijenga LH, et al. Successful treatment of low-grade oligodendroglial tumors with a chemotherapy regimen of procarbazine, lomustine, and vincristine. Cancer. 2005;103(4):802–9. Epub 2005/01/08. 10.1002/cncr.20828 . [DOI] [PubMed] [Google Scholar]
- 45.van den Bent MJ, Afra D, de Witte O, Ben Hassel M, Schraub S, Hoang-Xuan K, et al. Long-term efficacy of early versus delayed radiotherapy for low-grade astrocytoma and oligodendroglioma in adults: the EORTC 22845 randomised trial. Lancet (London, England). 2005;366(9490):985–90. Epub 2005/09/20. 10.1016/s0140-6736(05)67070-5 . [DOI] [PubMed] [Google Scholar]
- 46.Bauman GS, Ino Y, Ueki K, Zlatescu MC, Fisher BJ, Macdonald DR, et al. Allelic loss of chromosome 1p and radiotherapy plus chemotherapy in patients with oligodendrogliomas. International journal of radiation oncology, biology, physics. 2000;48(3):825–30. Epub 2000/10/06. . [DOI] [PubMed] [Google Scholar]
- 47.Douw L, Klein M, Fagel SS, van den Heuvel J, Taphoorn MJ, Aaronson NK, et al. Cognitive and radiological effects of radiotherapy in patients with low-grade glioma: long-term follow-up. The Lancet Neurology. 2009;8(9):810–8. Epub 2009/08/12. 10.1016/S1474-4422(09)70204-2 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
Data Availability Statement
The data is provided by the National Cancer Database and is available upon request by the National Cancer Database for investigators associated with Commission on Cancer accredited institutions. The National Cancer Database retains the decision of accessing the data based on an application process. Association to a Commission on Cancer accredited cancer program is required for applying. The application process opens throughout the year and requires a protocol submission. We applied for and were granted access to the data in the fall of 2017. Details on obtaining participant user files for data analysis is available in the following URL: https://na01.safelinks.protection.outlook.com/?url=https%3A%2F%2Fwww.facs.org%2Fquality-programs%2Fcancer%2Fncdb%2Fpuf&data=02%7C01%7Cjlvillano%40uky.edu%7C3e1f308e33a24f5e821608d60f427ddf%7C2b30530b69b64457b818481cb53d42ae%7C0%7C0%7C636713175589924852&sdata=siZFFXbVtzNa5qO4xWijysSJw3y%2FlA8uORwVyCub0C8%3D&reserved=0. Finally, collaborative interactions is an option for researchers outside of the Commission on Cancer accredited institutions, but data management and analysis (no data transfer) must be performed by the PI of the protocol approved from a Commission on Cancer accredited institution.