Abstract
Purpose
Diagnostic testing for dry eye disease (DED) in Sjogren's syndrome (SS) is well described. Little is published about monitoring this systemic autoimmune DED. We analyzed the SS related DED tests used in North American optometric practices and compared academic settings to private practice settings.
Methods
A retrospective chart review of 123 SS charts from 6 optometric practices in North America was conducted. Testing done during the first examination following a SS diagnosis was recorded on Research Electronic Data Capture (REDCap) database. The complete data file was reviewed and testing type and methodology were compared.
Results
Symptoms of DED (98.4% of charts),meibomian gland dysfunction (76.4% of charts), corneal staining with fluorescein (75.6% of charts) and anterior blepharitis (73.2% of charts) were the most frequently recorded variables. Clinicians used different methodologies to measure and grade these variables. Private practitioners were more likely to use symptom questionnaires and grading scales and to describe anterior blepharitis. Academic settings were more likely to record TBUT and tear meniscus height.
Conclusions
The monitoring of DED in SS is not uniform in optometric offices across North America. Creating accepted standards of testing will improve the ability of clinicians and researchers to communicate and understand the course of DED in SS.
Keywords: Sjogren's syndrome, Dry eye, Diagnostic tests, Ocular surface disease
Resumen
Objetivo
Las pruebas diagnósticas para la enfermedad del ojo seco en el síndrome de Sjogren (SS) están bien descritas. Se ha publicado poco acerca de la supervisión de este síndrome del ojo seco autoinmune sistémico. Analizamos el SS relacionado con las pruebas de ojo seco en las prácticas optométricas de Norte América, y comparamos los centros académicos con los centros de práctica privada.
Métodos
Se realizó una revisión retrospectiva de 123 historias clínicas de SS procedentes de 6 centros optométricos de Norte América. Las pruebas realizadas durante el primer examen, tras el diagnóstico de SS, se registraron en la base de datos Research Electronic Data Capture (REDCap). Se revisó el archivo de datos completo y se compararon el tipo de prueba y la metodología.
Resultados
Las variables más frecuentemente registradas fueron los síntomas de ojo seco (98,4% de las historias), disfunción de la glándula de Meibomio (76,4%), tinción corneal con fluoresceína (75,6%), y blefaritis anterior (73,2%). Los clínicos utilizaron diferentes metodologías para medir y clasificar dichas variables. Los facultativos privados tendieron a utilizar con mayor frecuencia los cuestionarios de síntomas y las escalas de clasificación, y a describir la blefaritis anterior. Los centros académicos tendieron a registrar con mayor frecuencia TBUT y la altura del menisco lagrimal.
Conclusiones
La supervisión del ojo seco en el SS no es uniforme en los centros optométricos de Norte América. La creación de estándares de pruebas aceptados mejoraría la capacidad de comunicar y comprender el curso del ojo seco en el SS por parte de clínicos e investigadores.
Palabras clave: Síndrome de Sjogren, Ojo seco, Pruebas diagnósticas, Enfermedad de la superficie ocular
Introduction
Sjogren's syndrome (SS) is a rheumatic autoimmune disease that is characterized by lymphocytic infiltration of the lacrimal and salivary glands, resulting in the hallmark symptoms of dry eye disease (DED) and dry mouth.1 The classification criteria for SS has changed rapidly since 2002.2, 3, 4
The variables used in these evolving criteria are standardized and include systemic measures of serum antibody markers, the histology of minor salivary glands, measurements of salivary flow and ocular measurements of symptoms, tear flow and ocular surface staining.2, 3
The ocular testing is well described. The American European Consensus Criterion (AECC) of 2002,2 describes ocular staining scores using a combined corneal and conjunctival staining score with rose bengal or fluorescein. The criterion requires a score of ≥4/9 in at least one eye that is the sum of the cornea, nasal and temporal conjunctiva graded 0–3 as described by van Bijsterveld.5 It also includes an assessment of DED symptoms that must be present for at least 3 months and that is graded on a visual analogue scale of 0–10.2
The American College of Rheumatology (ACR) criterion of 20123 described more precise staining grades: “if there are no punctate epithelial erosions (PEE) the score is 0. If 1–5 PEE are seen, the corneal score is 1; 6–30 PEE are scored as 2; and >30 PEE is scored as 3. An additional point is added if: (1) PEE occurred in the central 4 mm diameter portion of the cornea; (2) one or more filaments are seen anywhere on the cornea; or (3) one or more patches of confluent staining, including linear stains, are found anywhere on the cornea. The maximum possible score for each cornea is 6.”3
The ACR also describes the conjunctival staining score as follows: “grade 0 is defined as 0–9 dots of lissamine green staining of the interpalpebral bulbar conjunctiva (nasal and temporal bulbar conjunctivae graded separately); grade 1 is defined by the presence of 10–32 dots; grade 2 by 33–100; and grade 3 by >100 dots.” Symptoms are not standardized by the ACR criterion.
Although these various schemes are used for diagnosis, we questioned if the same testing was done when monitoring SS patients. Thus, the purpose of this paper is to analyze the results of a multi-centred retrospective SS chart review study to describe the customary practises identified in the 6 North American sites in the monitoring of DED in SS.
Methods
The chart review protocol was submitted to the Office of Research Ethics for each site. It was approved and conducted under a Waiver of Informed Consent. The study was designed in conformance with the ethical principles in the Declaration of Helsinki and with the ICH guidelines for Good Clinical Practice.
Only charts with a positive diagnosis of SS, that presented evidence of ongoing eye care in each practice, from the year 2000 onward, were included in this study. Although the AECC2 was not in place until 2002, it was used as the standard for diagnosis with which charts were included. The AECC requires, a minimum of 4 out of 6 of the following criteria with at least one of serum testing or lip biopsy to be positive.2
-
1.
Symptoms of dry eye for at least 3 months.
-
2.
Symptoms of dry mouth for at least 3 months.
-
3.
Signs of DED: Schirmer I score of ≤5 mm in 5 min and/or rose bengal or fluorescein staining score of ≥4/9 in at least one eye.
-
4.
Signs of dry mouth: salivary flow by unstimulated spitting in a cup of ≤1 ml in 5 min.
-
5.
Positive serum findings of autoantibodies ro and/or la.
-
6.
Positive salivary gland biopsy score: ≥1 focus score in 4 mm of tissue.
Charts for review were identified through diagnostic codes, doctor generated lists and rheumatology records at those sites with access to them. All sites also identified SS patients as they appeared for care during the study. Only first visit data, defined as the visit closest following the date of diagnosis and after January 1, 2000 was used. The charts were reviewed from October 2014 to June 15, 2015 at the 6 sites shown in Table 1.
Table 1.
Sites of this study.
| Site | # charts | Location | Type of practice |
|---|---|---|---|
| Site 1: University of Waterloo School of Optometry and Vision Science Clinic | n = 23 | Waterloo, Ontario | Academic |
| Site 2: Toronto Eye Care | n = 36 | Toronto, Ontario | Private Practice |
| Site 3: Eyelabs Optometry and Centre for Ocular Surface Disease | n = 9 | Brampton, Ontario | Private Practice |
| Site 4: Cornea Center for Clinical Excellence, Illinois College of Optometry | n = 19 | Chicago, Illinois | Academic |
| Site 5: Edmonds, Husz and Pemberton Eye Center | n = 19 | Tucson, Arizona | Private Practice |
| Site 6: UC Davis Health | n = 17 | Sacramento, California | Academic |
Each investigator entered data on RedCap (Research Electronic Data Capture) database. REDCap is a browser-based, metadata-driven EDC software solution and workflow methodology for designing clinical and translational research databases. Each investigator entered data for each variable using predetermined drop-down menus.
The researchers worked in teams and completed one chart at a time. The chart was opened and one researcher looked for the data within the chart while another entered the data. If there was a question about any entry the two researchers discussed the item and entered the appropriate information. Care was taken to distinguish missing data, not completed data and 0 value data.
The following variables were recorded from the initial visit:
-
1.
Demographics: age, sex, contact lens wear history
-
2.
Health history (i.e., presence of systemic diseases other than SS)
-
3.
Ocular health history
-
4.
Systemic medications:
-
a.
Drugs with known drying properties
-
b.
Other systemic medications
-
5.
Ocular topical medications: including use of lubricants
-
6.
Current DED treatments: including lid care, goggles, punctal plugs
-
7.
SS diagnostic criterion: including year of diagnosis and who made the diagnosis
-
8.
DED symptoms: included Standard Patient Evaluation of Eye Dryness II (SPEED II)6: AECC2 questionnaires with grading scales, other recorded symptoms
-
9.
Tear-break-up-time (TBUT) with fluorescein
-
10.
Corneal fluorescein staining: with various grading scales including AECC2 and ACR3
-
11.
Conjunctival staining: with various grading scales including AECC2 and ACR3
-
12.
The presence of superior limbic keratoconjunctivitis (SLK)
-
13.
Other DED results: including osmolarity, Schirmer and phenol red thread test
-
14.
Lid margin observations: including meibomian gland dysfunction (MGD), blepharitis, Demodex and telangiectasia
-
15.
Tear assessments – tear meniscus height and tear quality
Sites were divided into academic and private practice settings and the frequency of test usage was compared using Chi-square. p < 0.05 was considered statistically significant.
Results
Demographics and health history
All charts (n = 123) recorded age, sex and contact lens wear history. All charts included a generalized health history including SS diagnosis, co-morbidities and medications. All charts also contained ocular health history, ocular topical medications and use of DED treatments.
Dry eye symptoms
The symptom of dryness (Table 2) was recorded in some form in 98.4% (121/123) of charts. Although 46.3% (57/123) of charts had no grading scale for these symptoms, the following scales were used at various sites: AECC questionnaire2 (grade 0–10), qualitative assessment of mild, moderate, severe and SPEED II questionnaire6 (grade 0–28), (Table 3).
Table 2.
Percentage of charts with recorded symptoms.
| Site 1 | Site 2 | Site 3 | Site 4 | Site 5 | Site 6 | Totals | |
|---|---|---|---|---|---|---|---|
| Dryness | 23/23 (100%) | 36/36 (100%) | 9/9 (100%) | 18/19 (94.7%) | 18/19 (94.7%) | 17/17 (100%) | 121/123 (98.4%) |
| Irritation | 8/23 (34.7%) | 20/36 (55.5%) | 6/9 (66.7%) | 7/19 (36.8%) | 12/19 (63.1%) | 10/17 (58.8%) | 63/123 (51.2%) |
| Burning | 7/23 (30.4%) | 19/36 (52.8%) | 6/9 (66.7%) | 3/19 (15.8%) | 10/19 (52.6%) | 10/17 (58.8%) | 55/123 (44.7%) |
| Vision Problems | 6/23 (26.1%) | 19/36 (52.8%) | 6/9 (66.7%) | 11/19 (57.9%) | 15/19 (78.9%) | 16/17 (94.1%) | 73/123 (59.3%) |
| Photo-phobia | 4/23 (17.4%) | 20/36 (55.5%) | 7/9 (77.8%) | 2/19 (10.5%) | 9/19 (47.4%) | 10/17 (58.8%) | 52/123 (45.5%) |
Table 3.
Methodology of symptom collection.
| Site 1 | Site 2 | Site 3 | Site 4 | Site 5 | Site 6 | Total | |
|---|---|---|---|---|---|---|---|
| No system | 18/23 (78.2%) | 0 | 3/9 (33.3%) | 14/19 (73.7%) | 12/19 (63.1%) | 10/17 (58.8%) | 57/123 (46.3%) |
| Qualitative mild/mod/sev | 5/23 (21.7%) | 20/36 (55.5%) | 5/9 (55.5%) | 5/19 (26.3%) | 7/19 (36.8%) | 7/17 (41.2%) | 49/123 (39.8%) |
| AECC | 0 | 16/36 (44.4%) | 0 | 0 | 0 | 0 | 16/123 (13.0%) |
| SPEED II | 0 | 0 | 6/9 (66.7%) | 0 | 0 | 0 | 6/123 (4.9%) |
Abbreviations: AECC: American European Consensus Criterion; SPEED: standard patient evaluation of eye dryness; mod: moderate; sev: severe.
Schirmer test
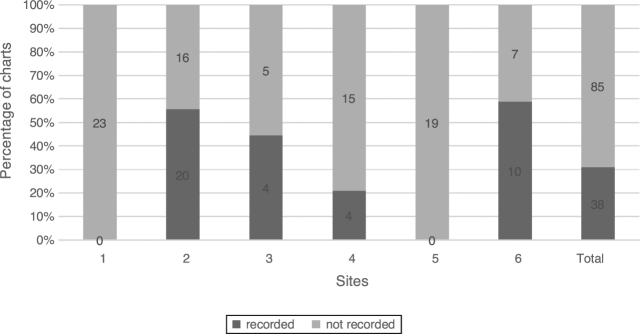
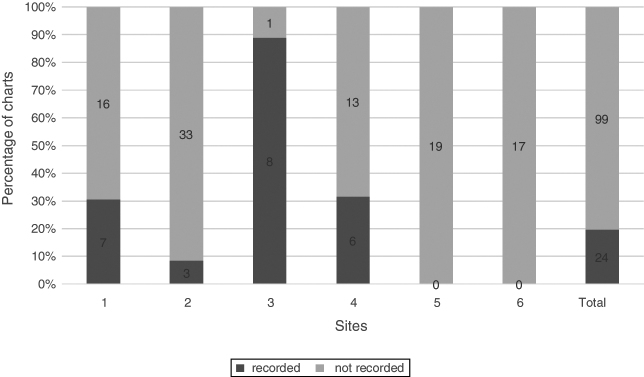
The Schirmer test was used at 4 sites and in 30.9% (38/123) of charts. The recording of Schirmer 1 test and Schirmer 2 test are combined in Fig. 1.
Figure 1.
Schirmer test. Percentage of charts with recorded Schirmer 1 and 2, n = 123.
Tear breakup time
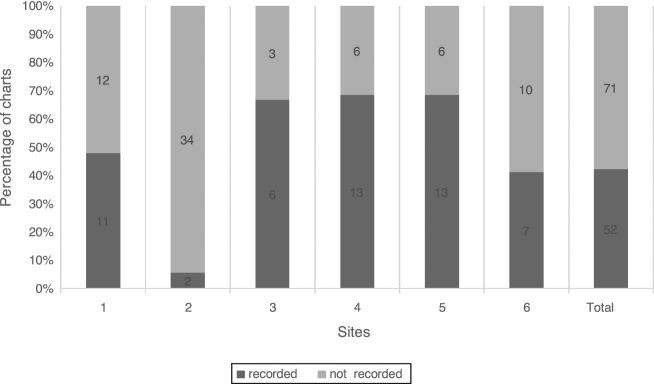
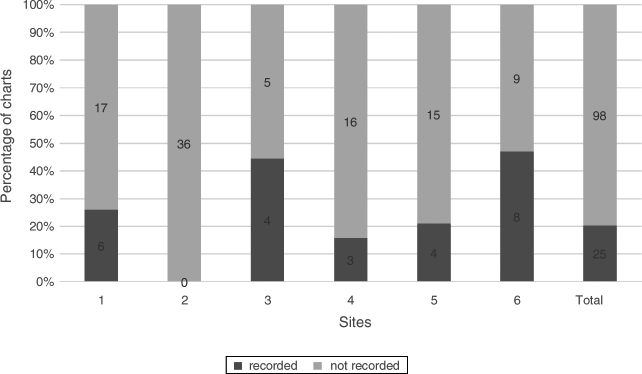
TBUT with fluorescein was recorded in at least one chart at all sites and in a total of 42.2% (52/123) of charts (Fig. 2).
Figure 2.
Tear breakup time. Percentage of charts with recorded TBUT, n = 123.
Corneal fluorescein staining
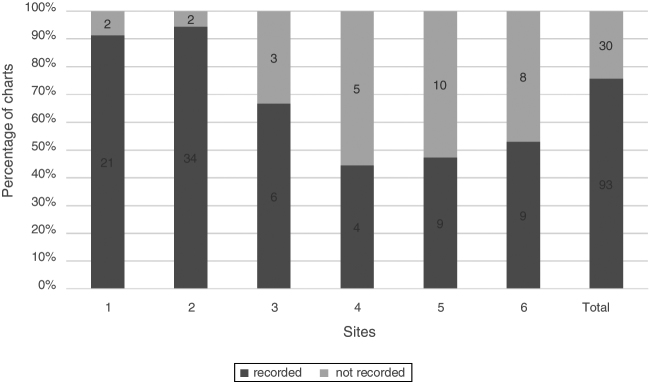
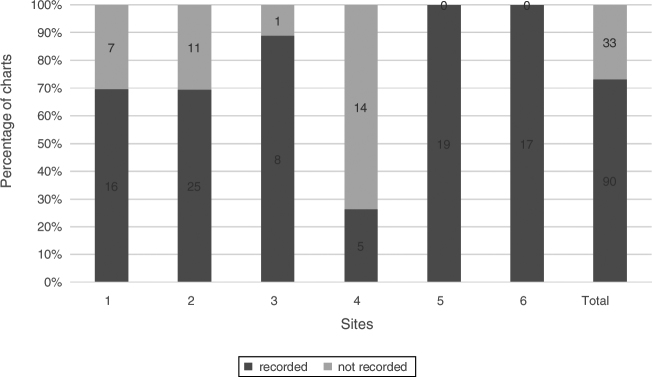
Corneal staining with fluorescein, a frequently recorded sign of DED, was found in 75.6% (93/123) of charts. Five of the six sites used a variety of stain descriptions that included region specification (central, nasal, temporal, superior, inferior), grading scale 1–4, extent of stain <25%, 25–50%, 50–75%, 75–100% and depth (micro, macro, coalesced). One site used the AECC2 (van Bijsterveld5) staining score of 0–3. Fig. 3 shows the percentage of charts with documentation of corneal staining.
Figure 3.
Corneal fluorescein staining. Percentage of charts with recorded corneal staining, n = 123.
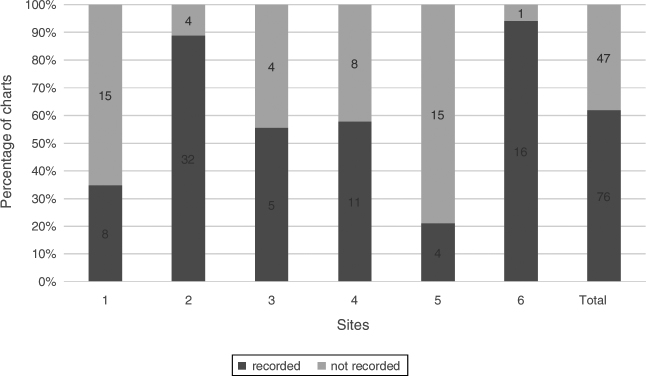
Conjunctival staining
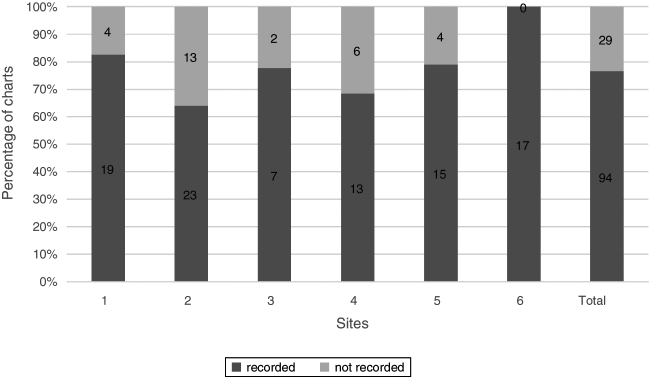
Conjunctival staining was recorded in 61.8% (76/123) of charts. Different dyes were used including: fluorescein, lissamine green and/or rose bengal. Four sites used a 0–4 scale, three sites used a qualitative scale of mild, moderate and severe and one site used the van Bijsterveld5 scale of 0–3 staining (Fig. 4).
Figure 4.
Conjunctival staining. Percentage of charts with recorded conjunctival staining, n = 123.
Tear quality
The recording of the quality of the tear film was done at 4 sites. 19.5% (24/123) of charts contained this documentation (Fig. 5). Entries included: bubbly, frothy and debris.
Figure 5.
Tear quality. Percentage of charts with recorded tear quality, n = 123.
Tear meniscus height
Tear meniscus height labelled normal or abnormal was recorded at 5 sites and in 20.3% (25/123) of charts (Fig. 6).
Figure 6.
Tear meniscus height. Percentage of charts with recorded meniscus height, n = 123.
Lid condition
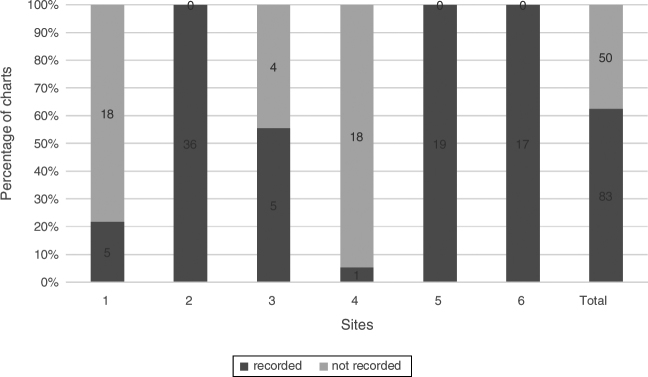
The recording of anterior blepharitis (Fig. 7) and MGD (Fig. 8) was carried out across all 6 sites. 73.2% (90/123) of charts documented observations of anterior blepharitis and 76.4% (94/123) of charts had documentation of MGD.
Figure 7.
Anterior blepharitis. Percentage of charts with recorded anterior blepharitis, n = 123.
Figure 8.
MGD. Percentage of charts with recorded MGD, n = 123.
The presence or absence of cylindrical dandruff/collarets on lid margin and lashes was recorded across all sites and was present in 67.5% (83/123) of charts (Fig. 9).
Figure 9.
Collarets on lashes. Percentage of charts with recorded collarets, n = 123.
Comparison of academic and private practice sites
In comparing academic and private practice sites (Table 4) with Chi square analysis, there were significant differences in protocols. Private practitioners were more likely to use DED symptom questionnaires and grading scales and to describe anterior blepharitis. Academic settings were more likely to record TBUT and tear meniscus height.
Table 4.
Comparison of academic and private practice sites.
| Academic sites (n = 59) | Private practice sites (n = 64) | Chi test significance | |
|---|---|---|---|
| No symptom scale | 42/59 (71.2%) | 15/64 (23.4%) | *p ≤ 0.001 |
| Use of DE questionnaires | 0/59 (0%) | 22/64 (34.3%) | *p ≤ 0.001 |
| Schirmer test | 14/59 (23.7%) | 24/64 (37.5%) | No significance |
| TBUT | 31/59 (52.5%) | 21/64 (32.8%) | *p ≤ 0.001 |
| Corneal stain | 44/59 (74.6%) | 49/64 (76.5%) | No significance |
| Conjunctival stain | 35/59 (59.3%) | 41/64 (64.1%) | No significance |
| Tear quality | 13/59 (22.0%) | 11/64 (17.2%) | No significance |
| Tear meniscus height | 17/59 (28.8%) | 8/64 (12.5%) | *p ≤ 0.001 |
| Anterior blepharitis | 38/59 (64.4%) | 52/64 (81.2%) | *p ≤ 0.001 |
| MGD | 49/59 (83.1%) | 45/64 (70.3%) | No significance |
Discussion
Our study demonstrates that the monitoring of DED in SS is not uniform across and between academic and private practice sites in North America. Of particular concern was the lack of standardized symptom assessment, wide differences in ocular surface stains and scales and lack of tear flow assessment.
The most commonly recorded DED tests were symptoms assessment, MGD, and corneal staining with fluorescein. With the exception of MGD assessment, these results agree with the work of Nichols et al.7 in 2000 when they reviewed 467 DED patient charts from non-private practice clinics. This demonstrates that little has changed in the past 18 years in the testing of DED.
Although DED symptoms were the most common chart entry, the absence of the use of standardized DED questionnaires was obvious as neither OSDI8 nor DEQ59 were used in these sites. One site did use the validated SPEED II questionnaire6 (Appendix A). Another site used the non-validated AECC questionnaire2 (Appendix B). Multiple symptoms of DED were not recorded with great frequency. Only the presence of dryness was consistently recorded in 97.6% (120/123) of charts.
Staining of the ocular surface is a vital aspect of grading DED disease.10, 11 Corneal fluorescein staining was recorded in 75.6% (93/123) of the charts and represents the most common objective recorded sign. The obvious problem in comparing our charts was the great variety of scales and descriptions.
Conjunctival staining was recorded with much less frequency at 61.8% (76/123) of the charts, but to some degree across all 6 sites. There was great variability in which stain and which grading scale was used. It is believed that fluorescein is not reliable in observing the conjunctiva unless a yellow wratten filter is employed.12 In the past, the most common stain for conjunctival evaluation was rose bengal13, 14 and is presently lissamine green.15 Rose bengal is rarely used today because of the level of stinging that it causes, and lissamine green is unavailable in some countries. Therefore, the conjunctiva may be ignored. However, factor analysis results suggest that corneal staining with fluorescein and conjunctival staining with rose bengal provide complementary yet separate information in diagnosing and monitoring DED.16 Therefore, two dyes are required for a thorough DED analysis in SS.
The infrequent use of tear flow and volume testing is important as SS related DED is considered to be a disease of reduced tear flow, secondary to lacrimal gland inflammation. The categorization and diagnosis of SS requires specific ocular testing, including Schirmer testing and ocular surface staining.2, 4 The visits included in this study followed the diagnosis and therefore the specific tests included in the diagnostic criteria were not mandatory. It appears that the clinicians who participated in this study, felt that monitoring SS related DED required a different set of tests. It is noteworthy that the Korb study17 that surveyed dry eye experts, suggested that Schirmer testing was the third most popular DED test following symptoms and corneal staining. Clearly that was not true in our study, particularly within academic settings. The new TFOS DEWSII18 suggests using tear meniscus height as a measure of tear volume and this test was used in only 20.3% (25/123) of charts.
Analysis of the tear film is also an important observation in DED.19 Interestingly, tear breakup time, considered a simple, routine test, was performed in only 42.3% (52/123) of charts with a range of 5.6–68.4% at the various sites. Both tear meniscus height and tear quality tests were recorded infrequently.
Observation of the lids, including meibomian gland function, is also important in DED assessment.20 The recording of MGD and anterior blepharitis was done across all sites with moderately high frequency at 76.4% (94/123) and 73.2% (90/123) respectively.
After reviewing the results of this study our researchers agreed that standardized testing for monitoring SS related DED would be ideal. In creating such a standard, the TFOS DEWSII report should be considered.11 For diagnosis, and after symptom assessment, the three most recommended tests are: non-invasive TBUT, osmolarity and ocular surface staining. The next recommended tests, tear meniscus height evaluation and meibomian gland and lipid layer assessment, help to distinguish evaporative versus aqueous deficient DED and have severity scales of mild, moderate and severe. If tear meniscus height is to substitute for Schirmer testing in SS DED, studies must be done to ensure that there is a correlation. Also, the scaling of corneal staining should be standardized. Sjogren's criteria require the van Bijsterveld score21 while the TFOS DEWS II report does not specify a preferred scale.11
We did observe differences in academic and private practitioner offices but even within these groups there were marked differences in testing, highlighting the fact that a good system for monitoring SS related DED does not exist. It is our belief that the TFOS DEWSII recommended testing should be applied to a large group of SS patients to test the validity of this proscribed testing in this unique group of dry eye patients.
Conclusions
Optometric practices in North America do not use a standardized method of monitoring SS related DED. Since studies that will help to describe the natural history of DED in SS will require standardization of both the diagnostic and monitoring visits, we suggest an application of the DEWSII diagnostic testing protocol in SS patients to understand its applicability in this unique DED group.
Conflicts of interest
The authors have no conflicts of interest to declare.
Acknowledgements
Peter Bergenske OD, FAAO and Robin Chalmers OD, FAAO for guidance, to Fellows Doing Research SIG of the American Academy of Optometry for resources and to CCLR renamed the Centre for Ocular Research and Education (CORE) for database development.
Funding was received from Canadian Sjogren's Syndrome Society and from Labtician.
Appendix A. SPEED II Questionnaire
Appendix B. The American European Consensus Criterion Dry Eye Questionnaire
References
- 1.Fox R., Stern M., Michelson P. Update in Sjögren syndrome. Curr Opin Rheumatol. 2000;12:391–398. doi: 10.1097/00002281-200009000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Vitali C., Bombardieri S., Jonsson R. Classification criteria for Sjogren's syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61:554–558. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shiboski S., Shiboski C., Criswell L. American College of Rheumatology Classification Crieteria for Sjogren's syndrome: a data-driven, expert concensus approach in the Sjogren's International Collaborative Clinical Alliance. Arthritis Care Res. 2012;64:475–487. doi: 10.1002/acr.21591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shiboski C.H., Shiboski S.C., Seror R. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjogren's syndrome: A consensus and data-driven methodology involving three international patient cohorts. Ann Rheum Dis. 2017;76:9–16. doi: 10.1136/annrheumdis-2016-210571. [DOI] [PubMed] [Google Scholar]
- 5.Van Bijsterveld O. Diagnostic tests in the sicca syndrome. Arch Ophthalmol. 1969;82:10–14. doi: 10.1001/archopht.1969.00990020012003. [DOI] [PubMed] [Google Scholar]
- 6.Ngo W., Situ P., Keir N., Korb D., Blackie C., Simpson T. Psychometric properties and validation of the Standard Patient Evaluation of Eye Dryness questionnaire. Cornea. 2013;32:1204–1210. doi: 10.1097/ICO.0b013e318294b0c0. [DOI] [PubMed] [Google Scholar]
- 7.Nichols K., Nichols J., Zadnik K. Frequency of dry eye diagnostic test procedures used in various modes of ophthalmic practice. Cornea. 2000;19:477–482. doi: 10.1097/00003226-200007000-00015. [DOI] [PubMed] [Google Scholar]
- 8.Schiffman R., Christianson M., Jacobsen G., Hirsch J., Reis B. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol. 2000;118:615–621. doi: 10.1001/archopht.118.5.615. [DOI] [PubMed] [Google Scholar]
- 9.Chalmers R., Begley C., Caffery B. Validation of the 5-item dry eye questionnaire (DEQ-5): discrimination across self-assessed severity and aqueous tear deficient dry eye diagnoses. Cont Lens Anterior Eye. 2010;33:55–60. doi: 10.1016/j.clae.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 10.Bron A., Abelson M., Ousler G. Methodologies to diagnose and monitor dry eye disease: report of the epidemiology subcommittee of the International Dry Eye Workshop. Ocul Surf. 2007;5:108–152. doi: 10.1016/s1542-0124(12)70083-6. [DOI] [PubMed] [Google Scholar]
- 11.Wolffsohn J., Arita R., Chalmers R. TFOS DEWS II management and therapy report. Ocul Surf. 2017;15:539–574. doi: 10.1016/j.jtos.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Eom Y., Lee J., Keun Lee H., Myung Kim H., Suk Song J. Comparison of conjunctival staining between lissamine green and yellow flitered fluorescein sodium. Can J Ophthalmol. 2015;50:273–277. doi: 10.1016/j.jcjo.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 13.Norn M. Dessication of the precorneal film I. Corneal wetting-time. Acta Ophthalmol (Copenh) 1969;47:865–880. doi: 10.1111/j.1755-3768.1969.tb03711.x. [DOI] [PubMed] [Google Scholar]
- 14.Norn M. Vital staining of the cornea and conjunctiva. Acta Ophthalmol (Copenh) 1973;51:483–491. doi: 10.1111/j.1755-3768.1973.tb06027.x. [DOI] [PubMed] [Google Scholar]
- 15.Manning F., Wehrly S., Foulks G. Patient tolerance and ocular surface staining characteristics of lissamine green versus rose bengal. Ophthalmology. 1995;102:1953–1957. doi: 10.1016/s0161-6420(95)30769-5. [DOI] [PubMed] [Google Scholar]
- 16.Caffery B., Simpson T., Wang S. Factor analysis of the clinical characteristics of Sjogren's Syndrome. Optom Vis Sci. 2010;87:742–750. doi: 10.1097/OPX.0b013e3181f32196. [DOI] [PubMed] [Google Scholar]
- 17.Korb D. Survey of preferred tests for diagnosis of the tear film and dry eye. Cornea. 2000;19:483–486. doi: 10.1097/00003226-200007000-00016. [DOI] [PubMed] [Google Scholar]
- 18.Wolffsohn J., Arita R., Chalmers R. TFOS DEWS II Diagnostic Methodology report. Ocul Surf. 2017;15:539–574. doi: 10.1016/j.jtos.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Lemp M., Baudouin C., Baum J. The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the international Dry Eye Workshop (2007) Ocul Surf. 2007;5:75–92. doi: 10.1016/s1542-0124(12)70081-2. [DOI] [PubMed] [Google Scholar]
- 20.Nichols K., Foulks G., Bron A. The international workshop on meibomian gland dysfunction:executive summary. Invest Ophthalmol Vis Sci. 2011;52:1922–1929. doi: 10.1167/iovs.10-6997a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Bijsterveld O. Diagnostic tests in the sicca syndrome. Arch Ophthalmol. 1969;82:10. doi: 10.1001/archopht.1969.00990020012003. [DOI] [PubMed] [Google Scholar]