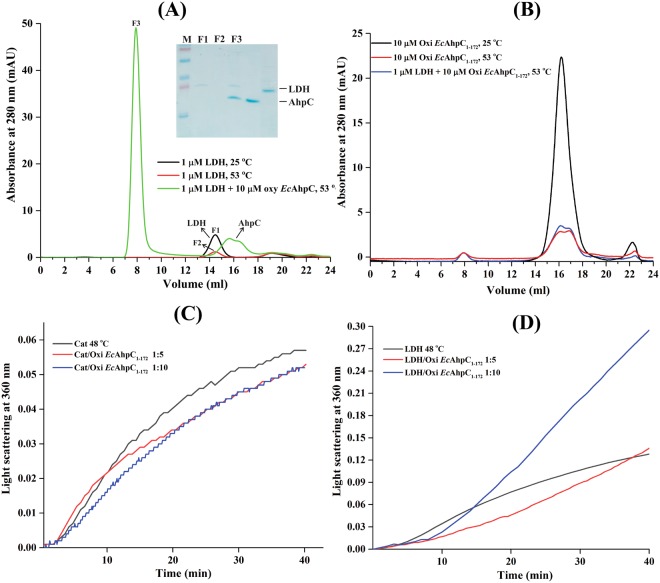
Figure 3.
Role of the C-terminal tail on heat induced oligomerization. (A) SEC analysis showed that 1 µM of LDH largely aggregated and that only a small amount remained soluble after heat treatment at 53 °C for 1 h (red) compared to that of at 25 °C (black). In comparison, SEC and SDS-PAGE (inset; full-length gel is presented) analysis revealed that the denatured LDH remained completely soluble after treatment at 53 °C for 1 h with HMW oligomers of oxidized EcAhpC. (B) The C-terminal tail deletion mutant, EcAhpC1–172, was largely precipitated after heat treatment at 53 °C in absence (red) and presence (blue) of LDH. The light scattering experiment showed that oxidized EcAhpC1–172 did not prevent the heat induced aggregation of (C) 1 µM catalase and (D) 1 µM LDH at 48 °C.