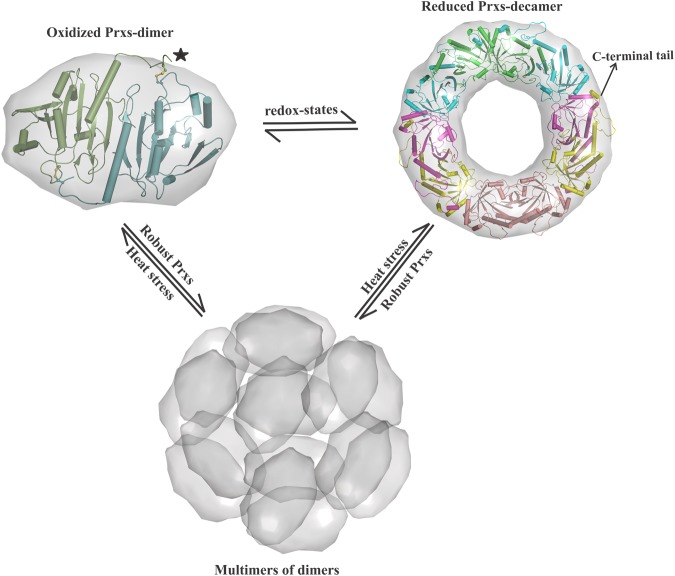
Figure 5.
Proposed chaperoning mechanism of AhpC/Prx1 enzymes. AhpC/Prx1 subfamily enzymes undergo redox-state dependent oligomerisation during their catalytic cycle. The decamer formed in the reduced state destabilises upon the formation of intermolecular disulphide bonds. In their reduced form, the C-terminal tail of Prx1 enzymes is required for their sensitivity to over-oxidation. The robust bacterial Prxs provide chaperon activity in the oxidized and reduced form, highlighting that the dimeric form can assemble into HMW oligomers under heat stress condition. The C-terminal tail of these enzymes is highly disordered in the oxidized structure, but essential for the heat induced HMW oligomer formation.