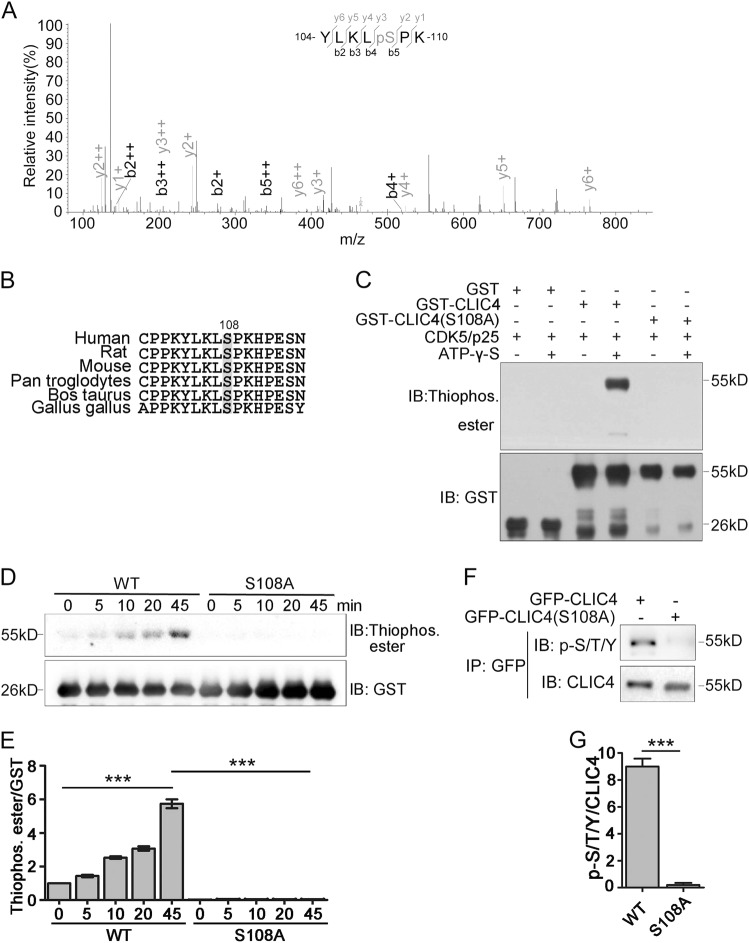
Fig. 2. CDK5 phosphorylates CLIC4 at serine 108.
a Purified GST-CLIC4 was phosphorylated by purified His-CDK5/p25 in vitro and analyzed by mass spectrometry. Mass spectrometric analysis was performed of a tryptic fragment matched to the +2 charged peptide 104-YLKLSPL-110; the results suggested that S108 was phosphorylated. The Mascot score was 27.5, and the expectation value was 1.39e−01. b Serine 108 and CDK5 phosphorylation consensus motif of CLIC4 are conserved in different species. c Immunoblotting of in vitro kinase assay performed by adding GST tag and recombined GST-CLIC4 or GST-CLIC4 (S108A) proteins into kinase assay buffer containing His-CDK5/p25 and ATP-γ-S. Anti-Thiophosphate ester antibody was used in western blotting to indicate the phosphorylated proteins. Anti-GST antibody was used to indicate the total proteins. d, e In vitro kinase assay in time course performed as described in c and reacted for different times as indicated. Thiophosphate ester/GST was quantified in e (n = 3 experiments). f, g Global phosphorylation levels of GFP-CLIC4 and GFP-CLIC4 (S108A) in cells. GFP-tagged proteins were precipitated with anti-GFP antibody from lysates of N2a cells transiently transfected with GFP-CLIC4 or GFP-CLIC4 (S108A). Samples were subjected to immunoblotting for p-S/T/Y and CLIC4. P-S/T/Y/CLIC4 was quantified in g (n = 3 experiments). Data are presented as the mean and SEM, and were analyzed by two-way ANOVA test (e) or unpaired Student’s t-test (g). *P < 0.05; **P < 0.01; ***P < 0.001