Abstract
Background: Data on long term use of Ayurvedic drugs is sparse. They may prove useful if combined with modern medicine in certain clinical situations (integrative medicine). We present the results of a long term observational study of RA-1 (Ayurvedic drug) used in the treatment of rheumatoid arthritis (RA).
Objective
The objective was to study safety of long term use of RA-1 for treatment of rheumatoid arthritis (RA).
Materials and methods
On completion of a 16 week randomized controlled study, 165 consenting volunteer patients were enrolled into a three year open label phase (OLP) study. Patients were symptomatic with persistent active disease and naïve for disease modifying anti-rheumatic drugs (DMARD). 57 patients were on fixed low dose prednisone. Patients were examined every 10–14 weeks in a routine rheumatology practice using standard care norms. They continued RA-1 (Artrex ™, 2 tablets twice daily) throughout the study period and were generally advised to lead a healthy life style. Based on clinical judgment, rheumatologist added DMARD and/or steroids (modified if already in use) to patients with inadequate response; chloroquine and/or methotrexate commonly used. Treatment response was assessed using American College of Rheumatology (ACR) efficacy measures and ACR 20% improvement index standard update statistical software (SAS and SPSS) were used; significant at p < 0.05.
Results
158, 130 and 122 patients respectively completed evaluations at 1, 2 and 3 year primary end point. The ACR 20 response (range 34–40%) remained stable over three years (p = 0.33). Patients improved optimum for several measures by one year (p < 0.05) and this was sustained. The use of steroids varied from 42 to 49% patients at yearly end points (mean daily dose 5 mg prednisone); correspondingly the use of DMARD varied from 20 to 34% patients. 40% patients on RA-1 did not require DMARD/steroids for control of disease. 77% patients reported adverse events, albeit mild and mostly gut related, and not causing withdrawal. Several study limitations (especially self-selection) were reduced by the high patient retention and consistency in drug use.
Conclusion
RA-1 is safe and effective in the long term management of symptomatic active chronic RA. DMARDs and/or steroids can be used judiciously along with RA-1 to treat difficult disease/flares. Further studies are required to evaluate RA-1 in early RA. This paves way for research and application of integrative therapeutic approach in clinical medicine.
Keywords: Rheumatoid arthritis, Treatment, Ayurveda, Herbal drug, Integrative medicine
1. Introduction
Ayurveda is an ancient medicinal system and is popularly practiced in India [1], [2], [3]. The holistic treatment approach combines herbomineral formulations and lifestyle changes. Ancient classic texts are important references. Though in use for centuries, effectiveness in the modern context needs validation [4], [5]. Plant based formulations are difficult to standardize [6]. Several research publications have attempted to unearth scientific evidence of efficacy of Ayurveda derived drugs to treat arthritis [7], [8], [9], [10], [11], [12], [13]. Cochrane reviews include a protocol on validation of Ayurvedic drugs in the treatment of RA [14], [15].
We reported the efficacy and safety of RA-1, a standardized Ayurvedic drug, in the treatment of active symptomatic RA in a 16 week randomized placebo controlled drug trial [7]. Patients were naïve for disease modifying anti-rheumatic drugs (DMARD). 40% patients were permitted to continued stable fixed daily low dose (< 7.5 mg daily) prednisone. This maiden landmark study drew attention towards the therapeutic potential of a standardized Ayurvedic medicine. Inspite of the improvement in the American College of Rheumatology (ACR), 20% response (primary efficacy) was not statistically significant and RA-1 performed better than the placebo in every efficacy variable with significant (p < 0.05) reduction in joint swelling and rheumatoid factor titer. Encouraged by the results, this study was undertaken as a three year open label phase (OLP) study.
Though doctors in India often combine Ayurvedic medicines and modern medicines in clinical practice, there is little scientific validation of this approach. It is prudent to add that to a large extent this kind of practice is surreptitious and unregulated. However, there is a growing enthusiasm for evidence-based integrative medicine [16] to treat difficult disorders such as RA.
In the current OLP, an integrative therapeutic strategy combining modern medicines and Ayurvedic drug (RA-1) was used to treat patients with inadequate response. The patient retention rate was more than 70% on study completion. We describe the effectiveness and safety of RA-1 in the treatment of RA in the current report.
2. Materials and methods
2.1. Site
The study was carried out at the Center for Rheumatic Diseases (CRD), Pune, in the Western India State of Maharashtra [17]. CRD is a community-based standard of care facility for rheumatic diseases.
2.2. Study design
This was a prospective observational study of three years following a protocol driven randomized study [7]. The OLP was guided by the overarching requirements of a true to life clinical practice. Patients were not segregated. Standard efficacy measures recommended by the ACR were used [18]. Patients were examined at 12–16 weeks intervals for efficacy and safety. All clinical services and RA-1 were provided free of cost to the patients. Patients were encouraged to continue RA-1 throughout the study period. Any worsening of symptoms and flares or persistent synovitis (inadequate response to RA-1) was managed by rheumatologists as per rheumatology practice norms. Rheumatologists added analgesics/NSAIDs (short term use), steroids and DMARD to a background RA-1 medication. All treatment decisions were made based on clinical judgment. The study design was approved by the CRD Ethics Committee.
2.3. Patient selection
165 consenting volunteer patients from the earlier randomized study were enrolled in the OLP [7]. Patients had active symptomatic disease and satisfied ACR classification criteria 1987 (randomization phase) (Table 1) [19].
Table 1.
Long term follow up study of patients suffering from rheumatoid arthritis (RA) and treated with RA-1 (standard Ayurvedic formulation): Baseline demographics.
| Variable | Total (n = 182) |
|---|---|
| Mean age (yrs) | 45 |
| Sex (Female) | 152 (83.5) |
| Family history (RA) | 41 (22.5) |
| Mean disease duration (yrs) | 7 |
| Disease activitya | |
| Mild | 31 (19) |
| Moderate | 88 (48) |
| Severe | 71 (28) |
| Very severe | 9 (5) |
| Functional class (ACR) | |
| I | 39 (22) |
| II | 106 (58) |
| III | 37 (20) |
| Prednisolone use (5.3 mg/day) | 76 (42) |
| Radiological erosions (hands) | 126 (69) |
| RF Seropositive | 149 (82) |
| ESR >60 mm/h | 65 (36) |
Based on Physician global assessment; n: total number of patients; ACR: American College of Rheumatology; RF: rheumatoid factor (nephlometry); Values in parentheses are percentages; see text for details.
2.4. Study and concomitant medication
RA-1 contained extracts of four medicinal plants- Withania somnifera (Ashwagandha), Boswellia serrata (Salai Guggul), Zingiber officinale (Shunti or ginger) and Curcuma longa (Haldi or circumin); Ayurvedic names shown in parenthesis. RA-1 was standardized and manufactured using modern pharmacological means [7]. Aqueous extracts were used for all plants except for guggul (aqua-alcoholic). The strength of plant extracts in each capsule of RA-1 was 90 mg Ashwagandha (root), 90 mg Shudh Salai Guggul (gum), 18 mg turmeric (rhizomes) and 24 mg ginger (rhizomes). In hindsight, we verify that the RA-1 formulation satisfied the CONSORT requirements (data not shown) [20]. Patients began with the optimum dose of RA-1 (investigational drug) which was 2 tablets (222 mg actives per tablet) twice daily following meals.
We followed current recommendations and our clinical practice norms while choosing modern medications and dosage schedule [21]. It was decided a priori to add steroids and/or DMARDs (oral chloroquine sulfate and/or methotrexate and/or sulfasalazine) to background RA-1 in patients with inadequate response or worsening of disease. Steroids were not to exceed 10 mg prednisone daily dose unless patient developed a systemic complication. We generally began with 5–10 mg prednisone daily and tapered to 2.5–5 mg daily once symptomatic improvement was sustained (6–8 weeks). We tried to stop steroids by a slow taper (1–2.5 mg every 2 weeks) if improvement was sustained for 6 months or so. Chloroquine sulfate was used in the dose of 250 mg daily. Oral methotrexate was begun at 10 mg single dose per week and was escalated up to 20 mg per week as per standard practice. The optimum dose of sulfasalazine was 2 gm daily in two divided doses. Patients continued concomitant drugs for coexisting diseases under supervision of their primary care physician.
In case of flare, patients were provided symptomatic relief with analgesics (paracetamol, tramadol) and/or NSAID (naprosyn, diclofenac, ibuprofen, nimesulide). The latter were used on pro rata basis or for short periods. NSAIDs were used sometimes round the clock for 4–8 weeks awaiting response from DMARD.
Patients were advised to maintain reasonable physical activity and fitness, reduce mental stress and consume healthy balanced diet. No specific advice was provided on any kind of diet or lifestyle changes.
2.5. Efficacy measures
In routine practice, all patients are recorded in a standard case record form which includes ACR core efficacy measures [18], [22]. A 68 joint count was used to record pain/tenderness (JCPT) while 66 joints (hip excluded) were examined for swelling (JCSW). Both the physician and patients global assessment were recorded using a category scale (5 grades: asymptomatic = 1, mild = 2, moderate = 3, severe = 4, very severe = 5). Pain was recorded using a Visual Analog Scale (VAS) of 10 cm, anchored at 0 for no pain and 10 for maximum pain during the preceding 24 h. Functional assessment and quality of life (QoL) were carried out using a validated Indian modified version of Stanford Health Assessment Questionnaire (HAQ) and scores (0–24, higher score indicates greater disability) were reported [23], [24]. Patients also recorded the likely duration of early morning stiffness and were examined for grip strength (using a mercury manometer) and 50 feet walking time.
2.6. Response to treatment
The assessment was essentially clinical though we report ACR index improvement [25]. In the current analysis, a relapse was considered if patients showed 25% worsening in each of the two joint counts (pain/tenderness and swelling) and 3 of the 5 parameters used for ACR improvement index (pain, global assessment by physician and patient, HAQ and ESR).
2.7. Investigations
Comprehensive laboratory and other relevant investigations (including renal, hepatic and metabolic parameters and EKG) were carried out on enrollment and at every yearly evaluation end point. However, ESR (Westergren), blood total and differential cell counts, platelet count serum aminotransferases, and urine analysis tests were carried out every 8–12 weeks. Rheumatoid factor (RF, nephelometry), C-reactive protein (CRP, nephelometry) and Interleukin 6 assay (IL 6, cell based) were carried out at yearly end points.
2.8. Adverse reactions
A priori determined checklist contained common drug related side effects known in Ayurveda and those associated with DMARDs and steroids. Patients were encouraged to report any event they considered related to medication.
2.9. Data and statistical analysis
The data from both the arms (active and placebo) of the randomized phase was clubbed at randomized baseline and 16 week completion for analysis in the current study. The primary evaluation time points were at yearly interval: weeks 52, 104 and 156. However, data was collected through clinical and laboratory examinations every 12–16 weeks and entered into the analytical database arranged at 12 weeks intervals (data not shown). Standard statistical software packages (SAS/OS 2 and SPSS) were used for analysis.
158, 130 and 122 patients were evaluated for efficacy at 1 year, 2 years and 3 years end point respectively; correspondingly 145, 93 and 61 patients on only RA-1 were evaluated for safety. The change from week 0 and week 16 to week 52 was analyzed using unpaired t-test while the stability of efficacy outcomes between weeks 52 and 156 were assessed using a longitudinal growth model (SAS PROC GENMOD and SAS PROC MIXED). Marginal homogeneity test for within treatment changes was performed for certain ranked data e.g. patient/physician grades.
Results for ‘any post week 16’ shown in the current report (especially Tables) pertains to observations based on any of the examination visits during the study and not necessarily the primary yearly end point visits. Data is only shown for primary evaluation end points at 1, 2 and 3 years; other individual evaluation visits (12 weekly) are not shown.
Data on safety in the current report is derived from patients who were on RA-1 medication without any concomitant DMARD; patients on low dose steroids begun a priori were not excluded. No interim analyses were performed. All calculations of time period on RA-1 medication were derived from week 0 baseline (randomized). Analysis of variance (ANOVA) was used when comparing groups. Multivariate regression analysis was carried out to determine predictors of response (details below). Results of tests of significance at p equal or less than 0.05 (two tailed) were considered significant.
3. Results
Table 1 shows selected patient characteristics and variables at randomized baseline. Patients suffered from active symptomatic disease and had moderately severe functional restriction (HAQ). Patients were naïve for DMARDs.
3.1. Clinical effectiveness
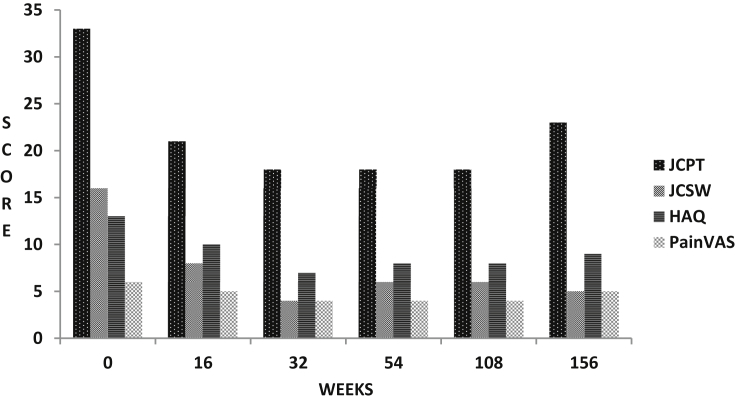
Patients had improved considerably during the randomized phase [7]. However, as is shown in Table 2 and Fig. 1, several patients remained symptomatic with active disease. There was further improvement in the OLP (Table 2, Fig. 1). The improvement was maximum during the first year with several variables (painful joints, swollen joints, pain and HAQ) showing significant change (p < 0.05). The improvement was sustained till three years and there was no significant change in trend. Significant consistent improvements were also recorded for hand grip strength and walking time (data not shown).
Table 2.
Three year follow up study of patients suffering from rheumatoid arthritis (RA) and treated with RA-1 (standard Ayurvedic formulation) following 16 week randomization phase: Clinical effectiveness data by evaluation end points (95% confidence interval shown in parenthesis for each mean/proportion).
| Measure/evaluation end points (weeks) | 0 (n = 182) | 16 (n = 165) | 52 (n = 158) | 104 (n = 130) | 156 (n = 122) |
| Mean pain/tender joint count (0–68) | 33.1 (30.4,35.4) | 20.08 (18.3,22.7) | 18.1 (15.5,20.1) | 18.2 (15.2,20.6) | 22.7 (19.0,26.4) |
| Mean swollen joint count (0–66) | 16.3 (14.9,17.8) | 7.7 (6.8,8.7) | 6.2 (5.1,7.0) | 6.2 (4.9,7.7) | 5.3 (4.1,6.4) |
| Mean pain visual analog scale (0–10 cm) | 5.9 (5.7,6.3) | 4.7 (4.4,5.0) | 4 (3.7,4.4) | 4.1 (3.8,4.6) | 4.6 (4.3,5.1) |
| Proportion (%) with patient global assess grade improve (1–5) | – | 45 (36.8, 51.2) | 51.1 (36.8, 51.2) | 52 (36.9, 52.9) | 60.1 (49.4,66.8) |
| Proportion (%) with physician global assess grade improve (1–5) | – | 63 (53.9, 68.1) | 59 (43.3, 57.8) | 64.2 (47.8, 63.8) | 48.1 (36.4, 53.9) |
| Mean health assess questionnaire (0–24) | 12.6 (11.7,13.4) | 9.6 (8.8,10.6) | 8 (7.1,8.8) | 8.2 (7.2,9.2) | 9.4 (8.2,10.4) |
| Proportion (%) with ACR 20 index improvement | – | 34.6 (27.7, 41.6) | 36.3 (29.3, 43.2) | 37.4 (29.6, 45.2) | 33.9 (25.5, 42.2) |
| Proportion (%) with ACR relapse index | – | – | 3.3 (0.7, 5.9) | 2.7 (0.1, 5.4) | 4 (0.6,7.5) |
Note: n: number of patients; 0 and week 16 pertain to randomization phase; 1 unit improvement from baseline considered for Patient & Physician global improvement [grades 1(asymptomatic)-5 (very severe)]; ACR: American College of Rheumatology; Proportion of patients (%) shown for patient and physician global assess improvement, ACR 20 index improvement and ACR relapse; ACR index are composite index based on joint counts, pain, patient and physician global assess, health assess questionnaire and erythrocyte sedimentation rate; See text for details.
Fig. 1.
Mean efficacy measures in patients of rheumatoid arthritis (RA) treated with supervised RA-1 (Ayurvedic drug) and followed for 3 years after an initial randomized placebo controlled phase (16 weeks); after 16 weeks patients with inadequate response also received supervised oral methotrexate and/or hydroxychloroquine and/or oral steroids (prednisolone ≤ 7.5 mg daily); JCPT: Joint Count for pain and tenderness (0–68); JCSW: Joint count for swelling (0–66); HAQ: CRD Version Indian Health Assessment Questionnaire (0–24); PainVAS: Pain Visual Analog scale (0–10 cm); range for measures shown in parentheses; see text for details.
The ACR 20 improvement response rate for the entire open label population was held steady with no significant change in the trend (p = 0.33); 40% at one year, 38% at two year and 34% at three year. In contrast, the ACR relapse rate was less than 5% at any of the study end points; ACR relapse rate was a composite index and is described in methods above (Table 2). 40% patients on RA-1 did not require DMARD/steroids for control of disease (Table 3).
Table 3.
Three year follow up study of patients (n = 182) suffering from rheumatoid arthritis (RA) and treated with RA-1 (standard Ayurvedic formulation) following 16 week randomization phase: Use of RA-1, low dose steroids and DMARD. Shows proportion of patients (percent).
| Drug/evaluation end points (weeks) | Any post week 16 | 0–16 | 16–52 | 52–104 | 104–156 |
|---|---|---|---|---|---|
| RA-1 Only | 38.2 | 58.2 | 46.4 | 52.4 | 47.5 |
| DMARD | 35.8 | 0 | 21.2 | 19.9 | 33.6 |
| Steroids | 55.5 | 41.8 | 45.5 | 41.8 | 45.9 |
| Any DMARD or steroid | 61.8 | 41.8 | 54.6 | 48.6 | 52.5 |
| Chloroquine | 18.8 | Nil | 7.9 | 6 | 13.8 |
| Methotrexate | 19.6 | Nil | 11.9 | 16.2 | 18.8 |
All patients received background treatment with RA-1; n: number of patients; 0 and week 16 pertain to randomization phase; low dose steroid pertain to equal or less than 7.5 mg daily prednisolone; DMARD include chloroquine sulfate, methotrexate and sulfasalazine; see text for details.
At least 10–15% patients reported absent symptoms or minimal disease at every evaluation visit after 24 weeks of RA-1 use; absent pain/tenderness and swelling in joints with pain VAS 2 cm or less and total HAQ score 3 and less (only few mild difficulties).
3.2. Concomitant DMARD and steroids
Table 3 shows the concomitant use of steroids and DMARD. Patients continued RA-1 as background medication. Overall, about 25% patients received steroids, 9% patients received DMARD and 28% received steroids plus DMARDs; inclusive of intermittent/brief use. Though the use of DMARD and steroids was somewhat higher in the third year, the trend in change was not significant (Table 3).
57 of the 78 patients who received prednisone during the randomized phase were continued in the OLP (Table 1). Not more than 50% patients were on steroid at any of the primary end point (Table 3). The mean daily dose was 4.7 mg at 1 year, 4.8 mg at 2 years and 5.1 mg at 3 years showing no significant change in trend. About 5% patients were found to have begun steroids at every yearly evaluation; correspondingly 2.5% at 1 year, 3% at 2 year and 10% at 3 years stopped steroids. Seven patients received intra-articular steroid (knee). Overall, we managed to stop steroids (and not restart later) in 14% patients on long term steroids; half the number of patients were being treated with only RA-1.
Patients begun on DMARD during the OLP were compared to patients who were treated with RA-1 monotherapy for selected variables at randomized baseline (Table 4). We selected the randomized baseline with the premise that patients who eventually required DMARD suffered from a more severe disease at the time of enrollment. We have not shown data at the actual time of beginning of DMARD. The institution of DMARD was driven by the clinical judgment of treating physician. There were no statistically significant differences between the two groups (Table 4). But the efficacy measures were certainly higher in patients begun on DMARD. A multivariable regression analysis employing several demographic variables, steroid use and efficacy measures (including lab) at baseline (Table 2) as dependent variables and DMARD use as independent variable was carried out. Only swollen joint count and pain VAS were found to be significant predictors of DMARD use and this supports the basic contention that patients begun on DMARD probably had a more severe disease to begin with (detail analysis not shown).
Table 4.
Comparing patients of RA treated with RA-1 and RA-1 plus DMARD at randomized baseline: mean (standard deviation) are shown (see text for details).
| Variable | RA 1 (n = 90) | RA 1 plus MTX (n = 50) | p (ANOVA) |
|---|---|---|---|
| Age (years) | 46.16 (11.02) | 42 (11.75) | 0.28 |
| Pain visual analog scale (cm) | 5.63 (2.35) | 6.24 (2.36) | 0.15 |
| Painful joint count (0–68) | 33.15 (18.3) | 33.81 (18.42) | 0.84 |
| Swollen joint count (0–66) | 15.82 (10.83) | 17.26 (11.30) | 0.46 |
| Health assessment questionnaire score (0–24) | 12.11 (6.33) | 13.38 (6.58) | 0.26 |
| ESR mm fall 1st hour | 53.27 (30.99) | 54.47 (34.01) | 0.83 |
| Hemoglobin gm/dl | 11.82 (1.51) | 11.94 (1.35) | 0.64 |
Table 3 also shows that the number of patients were almost equally divided between oral chloroquine and methotrexate. Considering DMARD use at any of the primary evaluation visits, 37% patients received chloroquine, 34% patients received methotrexate, 19% patients received a combination of chloroquine and methotrexate, 5% patients received sulfasalazine and 5% patients received a combination of methotrexate and sulfasalazine; single agent DMARD use was predominant. 15 patients (37%) in the DMARD group received DMARD only intermittently (up to 24 weeks).
3.3. Laboratory measures
Table 5 shows selected laboratory measures of disease activity and severity. The reduction in mean ESR (p = 0.003) at one year was sustained at two years but increased thereafter (p = 0.02). Mean serum CRP showed a significant reduction by year one (p < 0.0001); further data was unavailable. Serum Interleukin 6 showed a significant reduction during the OLP (p = 0.0002) but the response seemed to be inconsistent with other measures thereafter.
Table 5.
Three year follow up study of patients suffering from rheumatoid arthritis (RA) and treated with RA-1 (standard Ayurvedic formulation) following 16 week randomization phase: Laboratory (blood/serum assay) effectiveness data (mean value) by evaluation end points (95% confidence interval shown in parenthesis for each mean).
| Measures/evaluation end points (weeks) | 0 (n = 182) | 16 (n = 165) | 52 (n = 158) | 104 (n = 130) | 156 (n = 122) |
| ESR (mm) | 56.6 (51.4,60.9) | 57.4 (52.6,65.5) | 46.1 (40.4,51.7) | 45.2 (38.8,50.7) | 61.7 (54.4,68.2) |
| CRP (mg/dl) | 140.1 (114.4162.9) | 207.8 (160.0,250.4) | 83.8 (56.4,99.4) | nd | nd |
| Hemoglobin (gm/dl) | 11.76 (11.6,12.0) | 12.4 (12.2,12.6) | 11.6 (11.3,11.9) | 12.1 (11.8,12.4) | 12.2 (11.9,12.6) |
| Interleukin-6 (pg./ml) | 135.2 (118.3169.6) | 109.8 (89.5121.8) | 282.6 (212.9345.0) | 174.7 (136.7218.2) | 160.2 (119.7189.9) |
n: number of patients; 0 and week 16 pertain to randomization phase; nd: assay not done; ESR: erythrocyte sedimentation rate (Westergren, mm end 1st hour); CRP: C reactive protein; units shown in parenthesis with each measure; see text for details.
3.4. Adverse events (AE)
Table 6 shows the distribution of number of patients with AE at yearly interval. AE were counted in the period preceding the evaluation, for example, at year one, the AE pertain to the time interval between week 16 and week 52. During the randomized phase, 94% patients reported some AE; the corresponding frequency at one, two and three years was 68%, 92% and 100%. Overall, the pattern remained unchanged. Similar to the randomization phase, all AE were classified mild and required reassurance and symptomatic treatment. No patient ever withdrew due to the AE. The hematological and biochemical parameters remained within normal limits (data not shown). About 7% patients consuming only RA-1 recorded mild-to-moderate elevation (less than two times upper normal) in serum liver enzyme (alanine aminotransferase); other routine liver function tests remained normal and the increase in enzymes lasted less than 16 weeks. The dose of RA-1 was not reduced ever due to an AE.
Table 6.
Three year follow up study of patients (n = 182) suffering from rheumatoid arthritis (RA) and treated with RA-1 (Standard Ayurvedic formulation) following 16 week randomization phase: Adverse events/AE (proportion of patients-percent).
| Adverse event/evaluation end points (weeks) | Any post week 16 | 0–16 | 16–52 | 52–104 | 104–156 |
|---|---|---|---|---|---|
| Dysuria | 8.3 | 13.3 | 5.5 | 5.4 | 4.9 |
| Giddiness | 22.1 | 31.5 | 16.6 | 7.5 | 13.1 |
| Constipation | 33.1 | 21.0 | 12.4 | 33.3 | 36.1 |
| Acid Peptic Disorder | 37.9 | 25.4 | 13.1 | 40.9 | 42.6 |
| Pruritus | 46.2 | 29.8 | 21.4 | 50.5 | 42.6 |
| Mucositis | 38.6 | 26.5 | 17.9 | 45.2 | 47.5 |
| Insomnia | 37.2 | 38.7 | 17.9 | 43.0 | 41.0 |
| Anorexia | 51.0 | 45.3 | 31.0 | 49.5 | 50.8 |
| Nausea | 33.1 | 38.1 | 18.6 | 35.5 | 47.5 |
| Vomiting | 15.2 | 13.3 | 6.2 | 11.8 | 16.4 |
| Diarrhea | 17.9 | 12.2 | 2.8 | 15.1 | 19.7 |
| Other | 46.2 | 64.5 | 41.4 | 10.8 | 11.5 |
| Any AE | 77.2 | 90.6 | 68.3 | 92.5 | 100.00 |
n: number of patients; 0 and week 16 pertain to randomization phase; see text for details.
Common AE (> 25% patients) were anorexia, acid peptic related, nausea, mucositis (not necessarily mouth ulcers), vague episodic giddiness, and episodic pruritus (often nothing visible on the skin and sometimes dry skin), insomnia (mostly disturbed sleep). On comparison with the randomized phase, symptoms pertaining to anorexia, diarrhea, constipation, acid peptic dysfunction, mucositis and pruritus were increased in the OLP. Correspondingly, the frequency of insomnia, dysuria, giddiness, and nausea decreased in the OLP. None of the patients on long term steroids ever showed any Cushingoid features. None of the patients on chloroquine ever reported any ocular symptoms and less than 10% reported mild-to-moderate skin lesions (pigment changes and/or pruritus). The dose of weekly methotrexate remained in the range of 7.5–15 mg (median 10 mg); none showed any cytopenia or significant elevation (> 2 times upper limit normal) of serum liver enzymes. Less than 5 patients on methotrexate ever reported oral ulcers, hair loss, skin herpes zoster, respiratory and skin infections and increased hepatic enzymes (data not shown).
3.5. Extended open label phase
We continued to track patients though the attrition rate after 3 years was high and the patients were irregular. Though we provided free of cost consultation and laboratory services, the medicines had to be purchased. We believe that compliance to the medicines was not satisfactory at all times. However, we were able to comprehensively evaluate 55 patients and 32 patients at 5 years and 10 years respectively. Overall, we are encouraged to make some observations on the latter evaluation. About one third had followed with us diligently. About two thirds were in low disease activity/remission. About half the number had continued RA-1 over prolonged periods (up to 6 months at any one time). The use of DMARDs and/or steroids was intermittent (4–9 months at any one time). Patients reported use of analgesic/NSAID and steroids on a need basis or as per guidance of their family doctor. Overall, two third of patients made self decisions regarding beginning and stopping of drugs. Though the data was grossly limited, none of the patients reported any worrisome AE due to RA-1. We could also ascertain that at least 18 patients had died during this period and despite sketchy information the cause was apparently a severe infection (mostly pneumonia with multisystem complications) or a cardiovascular event (probably myocardial infarction/congestive cardiac failure).
4. Discussion
This three year open label extension phase study demonstrated considerable effectiveness and safety of RA-1 (Artrex™) in the long term management of patients suffering from active chronic RA. RA-1 was used to treat RA effectively as a single agent (monotherapy) in about 40% of the cohort. The remaining patients also in addition required steroids and or DMARD to control RA (Table 3). Steroids, albeit low dose, were used more frequently. Steroids could be stopped in 10% patients on long term use. RA-1 showed an excellent safety profile. None of the patients ever required any specific intervention/hospitalization or withdrew due to AE. We also demonstrated a novel integrative strategy of combining modern medicine and Ayurvedic drug. A strong community base, good patient retention rate and consistency in drug use were important factors that contributed towards the success of the study.
Patients with symptomatic, active and typical chronic disease were enrolled from the community (Table 1). 69% patients showed radiologically erosive disease. Patients were naïve for DMARD but several were maintained on fixed low dose of steroids prior to enrollment (Table 1). Though improved considerably during the randomized phase, several patients continued to suffer from symptomatic active disease (Table 2). We used a validated modified version of Stanford HAQ to capture the functional disability [24]. In our opinion, several of our lifestyle requirements like sitting on the ground impacts disease adversely and its measurement (HAQ) is critical towards the final outcome with any treatment. The Indian HAQ represents a greater challenge for therapeutic success. Patients demand that their preferential lifestyle be restored and HAQ response in the current study is a strong rejoinder for the effectiveness of RA-1.
Though RA-1 was superior to placebo for every study efficacy measure, the randomized phase failed to meet the primary efficacy criteria (ACR 20 response) [25]. The potential of the investigation drug was also realized in several animal (safety) and experimental studies (in-house data and not published). Thus, it was considered appropriate to further evaluate the effectiveness of RA-1. Also, the robust placebo response seen during the randomized phase was unlikely to influence a long term OLP. The current study design was to mimic real life modern rheumatology practice and as described above, there were several elements of a pragmatic design.
We presumed that patients with more severe disease will require steroids and/or DMARD but not statistically significant. Surprisingly this was not totally supported (Table 4). The baseline (randomized) measures were much higher in the patients who later required RA-1 plus DMARD. RA is a complex disease to measure and there are several domains of severity and progression which are difficult to capture. However, a regression analysis demonstrated that higher swollen joint count and pain VAS predicted use of DMARD; baseline use of steroid was not found significant (data not shown). Combining DMARD to RA-1 in patients with inadequate response to RA-1 seems to be a sensible option. Interestingly, the current study did not unravel a higher incidence of AE in patients with DMARD. But the toxicity profile of prolonged use of DMARD and/or steroids is well known [21]. We speculate that RA-1 may reduce the exposure to DMARD and/or steroids in some patients and make treatment safer.
The ACR 20 improvement index is used universally to differentiate a drug effect from placebo [25]. In the clinical context, this reflects modest improvement. ACR 50/70/90 improvement responses are more desirable and difficult to achieve. As a general statement, several controlled drug trials of reasonable duration (mostly 24 weeks) have demonstrated an ACR 20 in about 15–40% for patients only on methotrexate and 50–70% patients for the newer biological DMARDs with/without methotrexate, [26], [27]. By and large, biological DMARD have been evaluated in a setting of background methotrexate use and uncontrolled disease. Against this perspective, the ACR 20 response in the current study reflects modest to fair effectiveness. However, one time point ACR improvement response may not capture the total improvement and this is also well shown in the current study. The relapse rate used in the current study (see methods) was a strong measure of worsened disease/flare and was surprisingly very low (< 5%). Over 70% patients completed three year evaluation. This combined with other responses (HAQ, joint swelling and safety) suggest that the improvement with RA-1 was certainly much more than what ACR 20 indicates.
There was a slight drop in ACR 20 improvement response and a marginal increase in number of patients requiring steroid and/or DMARD at 3 years; trend of change not significant (Table 2). Patients were aware that they will lose study related concessions after 3 years and this may have effected compliance with medicines. A more serious issue would be whether RA-1 loses its efficacy over time? A longer period of study will be required to answer. In our opinion, this marginal worsening was artefactual and does not alter the conclusion regarding effectiveness of RA-1.
The safety of Ayurvedic medicines is borne by centuries of use and they are endearing to the community. The current study demonstrated an excellent safety profile of RA-1 (Table 6). Occasionally, our patients did attribute nausea, abdominal fullness, burning in epigastrium, anorexia and skin itching to RA-1. In our experience, digestive and acid-peptic related complaints are common in the community. In hind-sight, we should have captured similar baseline complaints of our patients which would have helped in understanding the AEs in the current study better. Some of the gut related AEs may be due to Ashwaganda and/or Guggul content in RA-1 [28], [29] . The patients in the current study often took modern and Ayurvedic medicines together in a short span of time following a meal. But none of the patients ever complained of reduction in relief or worsening of any symptom. Though we cannot comment with certainty on ‘drug interactions’ in the current study, it does not seem to be a problem. Patients were also monitored closely and none of the laboratory parameters ever suggested a worrisome hematological, hepatic, metabolic or renal alteration (data not shown).
Chronic RA is riddled with co-morbid disorders which often deteriorate with time [30]. Such disorders can be precipitated or worsened by a drug related AE. As an example, serious infections (often lung) are common in patients treated with methotrexate. We did not encounter such a situation in the current study.
RA-1 was rigorously standardized using modern pharmacological methods [7]. Although RA-1 formulation per se is not described in the Ayurveda texts, the constituent plant extracts are comprehensively described in Ayurvedic texts and have been evaluated for their anti-inflammatory and immunomodulatory properties (called ‘Rasayana’ in Ayurveda) in several experimental studies [28], [29], [31], [32], [33], [34], [35], [36], [37], [38]. Combination preparations are preferred and guided by complex Ayurvedic principles [39]. Several hundred Ayurveda formulations are currently sold over the counter and used in a manner similar to modern medicines. However, there are serious concerns about quality and standardization [40].
The concept of ‘reverse pharmacology pathway’ is well established for the clinical development and validation of Ayurvedic medicines [41]. This is an overarching strategy to validate the clinical effectiveness of Ayurvedic medicines upfront and in parallel investigate pharmacological properties and mechanisms of action. The clinical validation pathway is overwhelmingly encouraged. The argument for this approach stems from a viewpoint that centuries of use assures safety and tolerability of Ayurvedic medicines. This fast tract probably saves much time and money. We did adopt this strategy to a large extent for RA-1. However, several basic pharmacological and animal toxicity studies were carried out prior to initiating the clinical validation process of RA-1 [7].
While efficacy is supreme, nobody denies that safety and tolerability, access and affordability are major issues at least in developing countries. Biologic DMARDs are expensive and have major issues with infections (tuberculosis in particular) and malignancy. Their use is grossly limited in our setting though the situation may improve with advent of biosimilars [42]. But from a community perspective, drugs such as RA-1 hold tremendous socioeconomic appeal and are likely to be game changers.
RA is a prototypic autoimmune inflammatory systemic arthritis with the majority of patients showing a relentless progression which is also complicated by articular deformities and comorbidity (osteoporosis in particular). Patients die prematurely of atherosclerotic related cardiovascular events [30]. Based on clinical evidence in the current study, RA-1 provided long term good anti-inflammatory effect to treat RA. RA-1 also demonstrated long term effectiveness and especially improvement in function (HAQ) and this probably is more of a DMARD like effect. The ‘Rasayanic’ properties of RA-1 are likely to contribute to a DMARD effect. Rasayana in Ayurveda is akin to immune modulation in modern science and probably much more [2], [5], [31], [32]. A modest steroid sparing effect of RA-1 was shown in the current study. As speculated above, RA-1 is likely to reduce the need for DMARD. We encourage a further research agenda. RA-1 should be evaluated in the treatment of early RA and in patients showing inadequate response to methotrexate.
The current study had several limitations. Being open label, it was uncontrolled and riddled with several patient centric biases [43]. 122 (74%) out of 165 patients enrolled, completed three years and this restricts the problem of self-selection. It also fuels the success of our therapeutic adventurism with an integrative approach. We did not practice a holistic approach as advocated by Ayurveda. Addition of steroids and DMARD was an important confounding factor though we have carefully dissected the data to unravel the true effectiveness of RA-1. We used standard modern protocol and practice norms. However, in Ayurveda, the treatment also focuses on several other targets. As an example there is allegedly an improvement in digestive system, sleep pattern, mental and overall health but we did not look into these aspects.
Finally, we admit that we are late in the day for publishing this report. The study was completed several years ago. We wish to confirm to our readers that the delay was not due to any reason connected with the science or ethics of the current study. There were several other hurdles and priorities. Also, data entry and analysis took a long time. But we believe that the study is relevant. We hope to inspire our colleagues to seriously explore ancient ethnic medicinal system such as Ayurveda for better future therapies. This is also our legacy [44].
Conclusion
To conclude, RA-1 (Artrex ™), a standardized Ayurvedic herbal formulation, is an effective and safe DMARD in the long term management of RA. This study vindicates the results of the earlier randomized placebo controlled study [7]. Patients with inadequate response and or persistent active disease despite RA-1 benefited from a judicious concomitant use of steroids and DMARD. We strongly recommend clinical research in integrative medicine and with an initial focus on chronic non-communicable diseases like RA.
Sources of funding
Bioved Pharmaceuticals, Inc., USA provided an academic study grant and free of cost investigational product (RA-1). Bioved also engaged Averion International (Boston Biostatistics Inc.,) USA for a paid statistical analysis. The study was designed as a non-commercial investigator (AC) initiated study. The logistic, clinic and other data related support was provided by the Arthritis Research Care Foundation-Center for Rheumatic Diseases, Pune.
Conflict of interest
None.
Acknowledgement
Many people generously contributed their support, expertise and time to this very challenging study and we thank them all. We thank our patients and their relatives for participating in such a long term study. We acknowledge the support and contribution of the entire staff of Center for Rheumatic Diseases (CRD), Pune. We make special mention of our Ayurvedic physician colleague Dr Jayshree Patil. We thank Dr Phil Lavin, Senior Biostatistician (Boston USA) who played a pivotal role in the study design and carried out the earlier analysis (SAS). Prof S Sarmukkadam, Senior Biostatistician (BJ Medical College and Sassoon Hospital, Pune) helped in performing several analysis and we thank him wholeheartedly. To begin with, the entire process of development and validation of RA-1 was conceived by Prof Bhushan Patwardhan (University of Pune). And Bhushan has been a constant source of encouragement, knowledge and guidance to complete this study and we profoundly acknowledge his excellent contribution. Finally, the sponsorship and support provided by Ayurcore/Bioved USA, and the management of CRD Pune- Arthritis Research Care Foundation is gratefully acknowledged.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
References
- 1.Sharma R., Dash B. Chaukhambha Sanskrit Series Office; Varanasi, India: 2001. Charaka Samhita. [Google Scholar]
- 2.Chopra Arvind, Doiphode V.V. Ayurvedic medicine: core concept, therapeutic principles, and current relevance. Med Clin North Am. 2002;86:75–89. doi: 10.1016/s0025-7125(03)00073-7. [DOI] [PubMed] [Google Scholar]
- 3.Srikanta Murthy K.R. Chaukhambia Orientalia; Delhi: 1993. MadhavaNidanam (rogaviniscaya) of madhavakara (english translation) [chapter 22] [Google Scholar]
- 4.Chopra A., Patil J., Doiphode V., Patwardhan B. Exploring ancient ayurveda for rheumatology: traditional therapy, modern relevance and challenges. APLAR Bull. 2001;4:190–199. [Google Scholar]
- 5.Chopra A. Ayurvedic medicine and arthritis. Rheum Dis Clin North Am. 2000;26:133–144. doi: 10.1016/s0889-857x(05)70127-7. [DOI] [PubMed] [Google Scholar]
- 6.US FDA Guidance to industry on botanical drug products. CDER; June 2004. [Google Scholar]
- 7.Chopra A., Lavin P., Patwardhan B., Chitre D. Randomized double blind trial of an ayurvedic plant derived formulation for treatment of rheumatoid arthritis. J Rheumatol. 2000;27:1365–1372. [PubMed] [Google Scholar]
- 8.Chopra Arvind, Saluja M., Tillu G. Ayurveda–modern medicine interface: an overview and a critical appraisal based on experience with drug trials of standardized ayurvedic medicines to treat osteoarthritis (OA) and rheumatoid arthritis (RA) J Ayurveda Integr Med. 2010;1:190–198. doi: 10.4103/0975-9476.72620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Furst D.E., Venkatraman M.M., Krishna Swamy B.G., McGann M., Booth-Laforce C., Ram Manohar P. Well controlled, double-blind, placebo-controlled trials of classical Ayurvedic treatment are possible in rheumatoid arthritis. Ann Rheum Dis. 2011;70:392–393. doi: 10.1136/ard.2010.136226. [DOI] [PubMed] [Google Scholar]
- 10.Chopra Arvind, Saluja M., Tillu G., Venugopalan A., Sarmukaddam S., Raut A.K. Ayurvedic medicine offers a good alternative to glucosamine and celecoxib in the treatment of symptomatic knee osteoarthritis: a randomized, double-blind, controlled equivalence drug trial. Rheumatol Oxf. 2013;52:1408–1417. doi: 10.1093/rheumatology/kes414. [DOI] [PubMed] [Google Scholar]
- 11.Raut A., Bichile L., Chopra A., Patwardhan B., Vaidya A. Comparative study of amrutbhallatak and glucosamine sulphate in osteoarthritis: six months open label randomized controlled clinical trial. J Ayurveda Integr Med. 2013;4(4):229–236. doi: 10.4103/0975-9476.123708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soeken K.L., Miller S.A., Ernst E. Herbal medicines for the treatment of rheumatoid arthritis: a systematic review. Rheumatology (Oxford) 2003;42:652–659. doi: 10.1093/rheumatology/keg183. [DOI] [PubMed] [Google Scholar]
- 13.Kumar P.R.K. The efficacy of Ayurvedic treatment for rheumatoid arthritis: cross-sectional experiential profile of a longitudinal study. Int J Ayurveda Res. 2011;2:8–13. doi: 10.4103/0974-7788.83177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cameron M., Gagnier J.J., Chrubasik S. Herbal therapy for treating rheumatoid arthritis. Cochrane Database Syst Rev. 2011;16 doi: 10.1002/14651858.CD002948.pub2. [DOI] [PubMed] [Google Scholar]
- 15.Tillu G., Chopra A., Sarmukaddam S., Tharyan P. Ayurveda interventions for rheumatoid arthritis (Protocol) Cochrane Database Syst Rev. 2015;16 [Google Scholar]
- 16.Patwardhan B., Mutalik G., Tillu G., editors. Integrative approaches for health Amsterdam. Elsevier Academic Press; 2015. Advocacy for integration; pp. 1–26. [Google Scholar]
- 17.http://www.rheumatologyindia.org.
- 18.Felson D.T., Anderson J.J., Boers M., Bombardier C., Chernoff M., Fried B. The American College of Rheumatology preliminary core set of disease activity measures for rheumatoid arthritis clinical trials: the committee on outcome measures in rheumatoid arthritis clinical trials. Arthritis Rheum. 1993;36:729–740. doi: 10.1002/art.1780360601. [DOI] [PubMed] [Google Scholar]
- 19.Arnett F.C., Edworthy S.M., Bloch D.A., McShane D.J., Fries J.F., Cooper N.S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 20.Gagnier J.J., Boon H., Rochon P., Moher D., Barnes J., Bombardier C. Reporting randomized, controlled trials of herbal interventions: an elaborated CONSORT statement. Ann Intern Med. 2006;144:364–367. doi: 10.7326/0003-4819-144-5-200603070-00013. [DOI] [PubMed] [Google Scholar]
- 21.Singh J.A., Furst D.E., Bharat A., Curtis J.R., Kavanaugh A.F., Kremer J.M. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken) 2012;64:625–639. doi: 10.1002/acr.21641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ringold S., Singer N.G. Measures of disease activity in rheumatoid arthritis: a clinician's guide. Curr Rheumatol Rev. 2008;4:259–265. [Google Scholar]
- 23.Chopra A., Gore A., Paranjape S., Edmonds J. Program of the 8th APLAR Congress, Melbourne, Australia, April 21–25, 1996. ICMS Pty Ltd; Victoria, Australia: 1996. Modified health assessment questionnaire: an Indian study for validity and relevance. 87 (323) [Google Scholar]
- 24.Chopra A., Saluja M. Validation and usefulness of Indian version (CRD Pune) health assessment questionnaire: drug trials, community practice and COPCORD Bhigwan population study (1994–2012) Indian J Rheumatol. 2012;7:74–82. [Google Scholar]
- 25.Felson D.T., Anderson J.J., Boers M., Bombardier C., Furst D., Goldsmith C. American College of Rheumatology. Preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum. 1995;38:727–735. doi: 10.1002/art.1780380602. [DOI] [PubMed] [Google Scholar]
- 26.Orme M.E., MacGilchrist K.S., Mitchell S., Spurden D., Bird A. Systematic review and network meta-analysis of combination and monotherapy treatments in disease-modifying anti rheumatic drug-experienced patients with rheumatoid arthritis: analysis of American College of Rheumatology criteria scores 20, 50, and 70. Biologics. 2012;6:429–464. doi: 10.2147/BTT.S36707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Dell J.R. Treatment of rheumatoid arthritis. In: Firestein G.S., Budd R.C., Gabriel S.E., McInnes I.B., O'Dell J.R., editors. Kelley & Firestein's textbook of rheumatology. 10th ed. Elsevier (RELX India Pvt Ltd); New Delhi: 2017. pp. 1187–1212. [Google Scholar]
- 28.Mishra L.C., Singh B.B., Dagenais S. Scientific basis for the therapeutic use of Withaniasomnifera (ashwagandha): a review. Altern Med Rev. 2000;5:334–346. [PubMed] [Google Scholar]
- 29.Basch E., Boon H., Davies-Heerema T., Foppo I., Hashmi S., Hasskarl J. Boswellia: an evidence-based systematic review by the natural standard research collaboration. J Herb Pharmacother. 2004;4:63–83. [PubMed] [Google Scholar]
- 30.Scott D.L., Wolfe F., Huizinga T.W. Rheumatoid arthritis. Lancet. 2010;376:1094–1110. doi: 10.1016/S0140-6736(10)60826-4. [DOI] [PubMed] [Google Scholar]
- 31.Thatte U., Chhabria S., Karandikar S.M., Dahanukar S. Immuno-therapeutic modifications by Indian medicinal plants. Indian Drugs. 1987;25:85–87. [Google Scholar]
- 32.Rege N.N., Thatte U.M., Dahanukar S.A. Adaptogenic properties of six rasayana herbs used in Ayurvedic medicine. Phytother Res. 1999;13:275–291. doi: 10.1002/(SICI)1099-1573(199906)13:4<275::AID-PTR510>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 33.Ziauddin M., Phansalkar N., Patki P., Diwanay S., Patwardhan B. Studies on the immunomodulatory effects of Ashwagandha. J Ethnopharmacol. 1996;50:69–76. doi: 10.1016/0378-8741(95)01318-0. [DOI] [PubMed] [Google Scholar]
- 34.Abdel-Tawab M., Werz O., Schubert-Zsilavecz M. Boswelliaserrata- an overall assessment of in vitro, preclinical, pharmacokinetic and clinical data. Clin Pharmacokinet. 2011;50:349–369. doi: 10.2165/11586800-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 35.Chrubasik S., Pittler M.H., Roufogalis B.D. Zingiberisrhizoma: a comprehensive review on the ginger effect and efficacy profiles. Phytomedicine. 2005;12:684–701. doi: 10.1016/j.phymed.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 36.Mathy-Hartert M., Jacquemond-Collet I., Priem F., Sanchez C., Lambert C., Henrotin Y. Curcumin inhibits pro-inflammatory mediators and metalloproteinase-3 production by chondrocytes. Inflamm Res. 2009;58:899–908. doi: 10.1007/s00011-009-0063-1. [DOI] [PubMed] [Google Scholar]
- 37.Joshi K., Chavan P., Warude D., Patwardhan B. Molecular markers in herbal drug technology. Curr Sci. 2004;87:159–165. [Google Scholar]
- 38.Aggarwal B.B., Ichikawa H., Garodia P., Weerasinghe P., Sethi G., Bhatt I.D. From traditional Ayurvedic medicine to modern medicine: identification of therapeutic targets for suppression of inflammation and cancer. Expert Opin Ther Targets. 2006;10:87–118. doi: 10.1517/14728222.10.1.87. [DOI] [PubMed] [Google Scholar]
- 39.Gogate V.M. Ayurvedic pharmacology and therapeutic uses of medicinal plants—DravyagunaVidnyan. 1st English edn. Bharatiya Vidya Bhavan; Mumbai: 2000. Ayurvedic text on clinical pharmacology. [Google Scholar]
- 40.The Ayurvedic pharmacopoeia of India, vol. I: part I. Reprint. The Controller of Publications, Delhi. ISBN: 81-901151-3-8.
- 41.Patwardhan B., Vaidya A.D.B., Chorghade M. Ayurveda and natural products drug discovery. Curr Sci. 2004;86:789–799. [Google Scholar]
- 42.Chopra A., Subramanian S. Biosimilar DMARD in rheumatology: a general perspective with focus on India [Review] Indian J Rheumatol. 2012;7:89–96. [Google Scholar]
- 43.Higgins J.P.T., Altman D.G., Sterne J.A.C. Chapter 8:Assessing risk of bias in included studies. In: Higgins J.P.T., Green S., editors. Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated march 2011] The Cochrane Collaboration; 2011. www.cochrane-handbook.org Available from: [Google Scholar]
- 44.Valiathan M.S. Orient Longman; Chennai: 2003. The legacy of caraka. [Google Scholar]