FIG. 2.
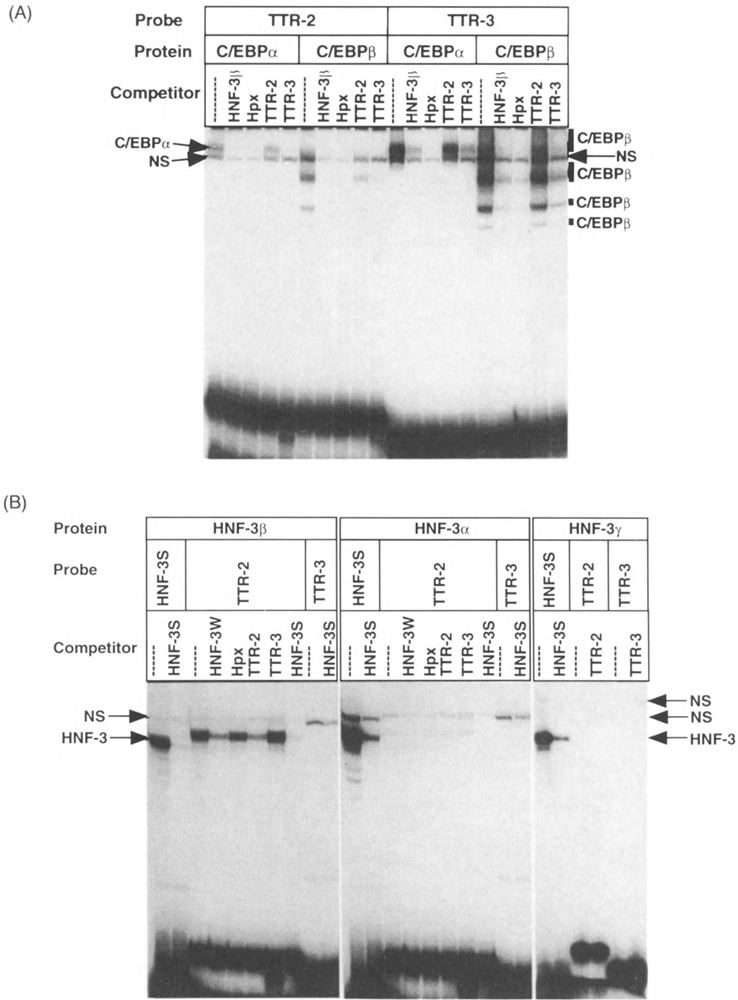
The TTR-2 sequence contains a divergent HNF-3 site that exclusively binds the HNF-3β isoform. (A) Recombinant C/EBPα and C/EBPβ proteins synthesized in HeLa cells were used for complex formation with the TTR-2 and TTR-3 oligonucleotides, and a 100-fold molar excess of the indicated competitors was included to examine binding affinities (see Fig. 1). This includes the HNF-3β promoter oligonucleotide (–97/–67), which binds both HNF-3 and C/EBP proteins (49,56), and the strong affinity C/EBP binding site from the hemopexin (Hpx) promoter (50). The position of the C/EBPα and C/EBPβ protein/DNA complexes and a nonspecific (NS) band that did not compete with C/EBP binding sites are also indicated. Control HeLa cell nuclear extracts did not contain any binding activity to the TTR-2 or TTR-3 oligonucleotides. (B) The TTR-2 sequence selectively binds the HNF-3/3 isoform. Recombinant HNF-3α, HNF-3β, and HNF-3γ proteins were used for complex formation with the TTR-2 and TTR-3 oligonucleotides and a 100-fold molar excess of the indicated competitors was included [HNF-3W is the same as HNF-3β in (A)]. The strong affinity HNF-3 site (HNF-3S) from the TTR promoter (15) was included to demonstrate that all of the recombinant HNF-3 isoforms were active for DNA binding activity and TTR-3 was used as a negative control. The position of the HNF-3 protein/DNA complexes (HNF-3) and several nonspecific complexes (NS) that were not inhibited by competition with HNF-3 binding sites are also indicated.
