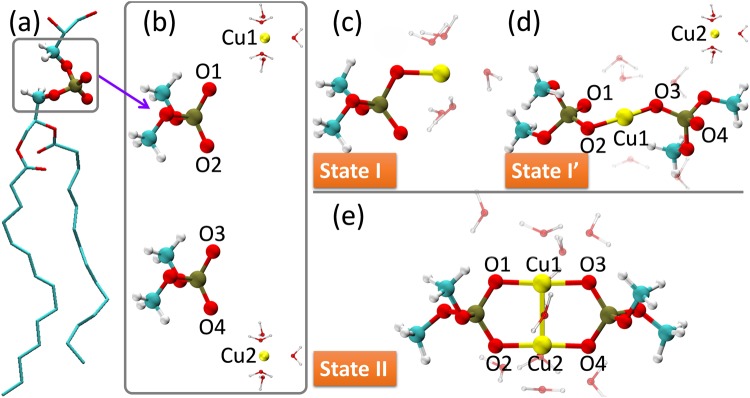
Figure 2.
Ways of two hydrated Cu2+ ions binding with two phospholipids. The cyan, brown, red, white and yellow balls represent carbon, phosphorus, oxygen, hydrogen and copper, respectively. (a) A phosphate group in a phospholipid (PL). (b–d) Hydrated Cu2+ ions binding to phospholipids based on a simplified model, H3C-[PO4]−-CH3. (b) Initial state. Four molecular groups (H3C-[PO4]−-CH3 and [Cu(H2O)5]2+) are separated by a large distance. (c) State I. Two Cu2+ ions bind to two phospholipids, respectively, resulting in two [PL-Cu(aq)]+ structures. (d) State I′. One Cu2+ ion binds to two phospholipids, forming a structure [PL-Cu(aq)-PL]. (e) State II. Two Cu2+ ions bind simultaneously with two phospholipids, forming a [PL-diCu(aq)-PL]2+ structure.