Fig. 2.
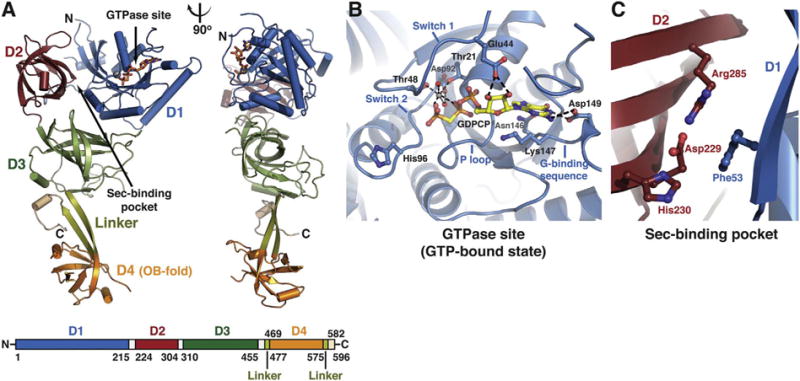
Structure of human eEFSec. (A) eEFSec folds into a chalice-like structure. The structure is shown as cartoon in two orientations that are related by 90° clockwise rotation around the vertical axis. Domains D1 (blue), D2 (red), and D3 (green) form a cup resembling EF-Tu. Flexible linker (light green) connects D3 with an appended D4 (orange), which represents the base of the cup. The C-terminal element (beige) folds below D3 (see: Fig. 3F). The GTPase site and the Sec-binding pocket are indicated with arrows. The domain arrangement and coloring scheme are summarized in a bar diagram below the cartoon diagram. (B) The close-up view of the GTPase site of eEFSec when complexed with a GTP analog. The main elements of the site are labeled and the major interactions between the amino-acid side chains, GTP analog, Mg2+ and water molecules (red spheres) are shown. (C) The close-up view of the Sec-binding pocket of human eEFSec. The pocket resides at the interface of D1 and D2. (For interpretation of the references to colour in this figure legend, the reader is referred to the online version of this chapter.)
