Fig. 4.
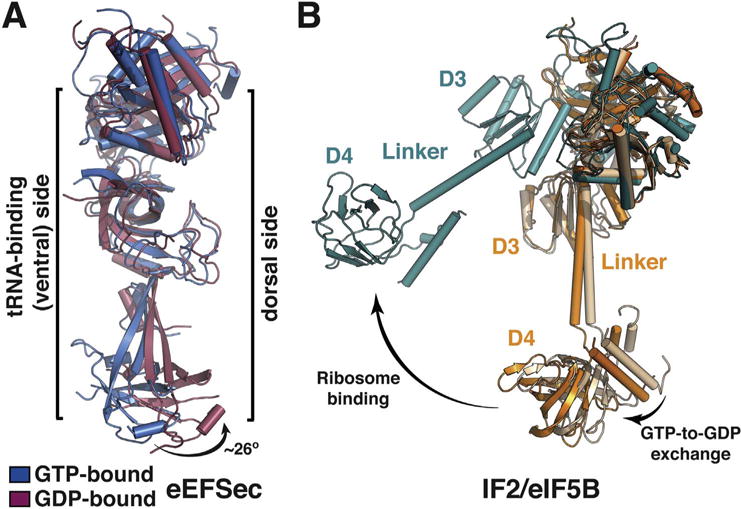
A major, non-canonical conformational change in eEFSec upon GTP-to-GDP exchange. (A) A side view of overlaid GTP- (blue) and GDP-bound (red) states of eEFSec shows that GTP hydrolysis induces a major conformational change in D4, which is distinct from the canonical situation in EF-Tu. In particular, D4 swings ~26° away from the tRNA-binding (or ventral) side of eEFSec and drags with it the linker region. D1 and D2 move towards and away from the tRNA-binding side. (B) The magnitude of the GTPase-coupled conformational change of IF2/eIF5B depends on the ribosome. In the absence of the ribosome, the movement of D3, linker, and D4 is relatively small (beige to orange), whereas the conformational change is quite pronounced when the factor binds to the ribosome (orange, or beige, to teal). (For interpretation of the references to colour in this figure legend, the reader is referred to the online version of this chapter.)
