Fig. 5.
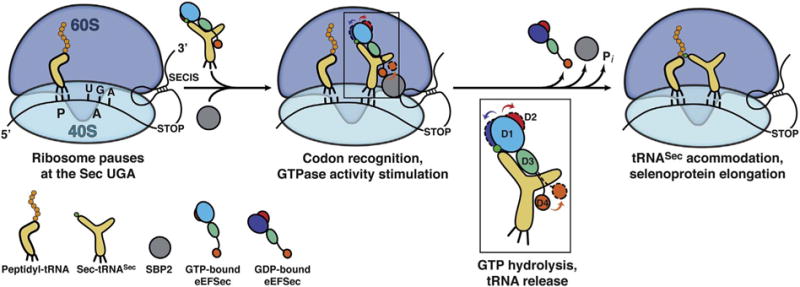
Proposed mechanism of elongation of selenoprotein synthesis as the Sec UGA codon. The 80S ribosome pauses at the SEC UGA codon. The peptidyl-tRNA is bound to the P site and is awaiting the next amino acid. The 3′-UTR SECIS element tethers a large complex composed of SBP2 (grey sphere), the GTP-bound state of eEFSec, and Sec-tRNASec near the ribosome. How eEFSec delivers Sec-tRNASec to the A site and what is the role of SBP2 and ribosomal elements during this step is still not clear. Formation of proper codon-anticodon interactions at the decoding center of the 40S subunit presumably stimulates the GTPase activity of eEFSec. GTP hydrolysis leads to a slight ratchet of D1 (shades of blue) and D2 (red), and a larger swing of D4 (orange). D1 moves towards the ventral (tRNA-binding) side of eEFSec, whereas D2 and D4 move in the opposite direction (see inset). The domain movements, which are different from the EF-Tu-based system, cause dissociation of eEFSec from Sec-tRNASec and the ribosome. This then allows accommodation of Sec-tRNASec within the PTC and the reaction of peptide bond synthesis to occur. The end result is an elongated nascent selenoprotein. (For interpretation of the references to colour in this figure legend, the reader is referred to the online version of this chapter.)
