Abstract
Pediatric hepatocellular carcinoma (HCC) is the second common malignant liver tumor in children after hepatoblastoma. It differs from the adult HCC in the etiological predisposition, biological behavior and lower frequency of cirrhosis. Perinatally acquired hepatitis-B virus, hepatorenal tyrosinemia, progressive familial intrahepatic cholestasis, glycogen storage disease, Alagille’s syndrome and congenital portosystemic shunts are important predisposing factors. Majority of children (87%) are older than 5 years of age. Following mass immunization against hepatitis-B, there has been a drastic fall in the incidence of new cases of pediatric HCC in the Asia-Pacific region. Management is targeted on complete surgical removal either by resection or liver transplantation. There is a trend towards improving survival of children transplanted for HCC beyond Milan criteria. Chemotherapeutic regimens do not offer good results but may be helpful for down-staging of advanced HCC. Surveillance of children with chronic liver diseases with ultrasound and alpha-fetoprotein may be helpful in timely detection, intervention and overall improvement in outcome of HCC.
Keywords: Hepatocellular carcinoma, Children, Risk-factors, Outcome, Liver transplantation
Core tip: The review elaborately describes various risk factors, treatment options and outcome of children with hepatocellular carcinoma.
INTRODUCTION
Hepatocellular carcinoma (HCC) is a rare malignancy of childhood affecting predominantly adolescent males with an overall poor survival. Both hepatoblastoma and HCC together account for 0.5%-1.5% of all childhood malignancies and 4% of all pediatric liver transplantations (LT)[1-4]. Hepatoblastoma constitutes about 67%-80% of all pediatric liver cancers worldwide, remaining 20%-33% are HCC. The proportion of HCC among pediatric liver cancers varies and depends on the geographical locale and prevalence of Hepatitis-B virus (HBV) infection in the community[2,3,5]. There are two distinct subsets of HCC in the pediatric age group - first, in the setting of cirrhosis or underlying metabolic, infectious or vascular liver disease, and second, sporadic HCC without preceding liver disease. Tyrosinemia and perinatally acquired HBV infection are two major risk factors[1,2,6,7]. Cirrhosis is absent in 26%-62% of childhood HCC[6,7]. Fibrolamellar variant of HCC is seen in 24% with a relatively better prognosis[8]. In the present review, we tried to elaborate various risk factors, therapeutic and preventive strategies and outcome of pediatric HCC.
ADULT VS PEDIATRIC HCC - ARE THEY DIFFERENT DISEASES?
Pediatric HCC differs from its adult counterpart in various aspects[9,10]. Adult HCC usually develops in the setting of chronic necro-inflammation going on for several years to decades secondary to alcohol, viral hepatitis-B or C or non-alcoholic fatty liver disease (NAFLD)[11]. Contrastingly, pediatric HCC develops either in a cirrhotic or non-cirrhotic background and inciting agent is either a toxic metabolite (tyrosinemia) or a mutated virus (HBV)[1,2,6]. Gender predilection, although has been shown to be present in studies from East, is not as marked as in adults[6-8,12-18]. White children are equally predisposed to develop HCC as blacks[6-8,15]. The Surveillance, Epidemiology, and End Results (SEER) database has shown a stable incidence of pediatric HCC in United States over last 4 decades, whereas in adults the incidence has almost tripled between 1975 and 2005 primarily because of hepatitis-C, and also contributed by hepatitis-B, alcohol, obesity and NAFLD[5,11]. Fibrolamellar variant of HCC (FL-HCC) comprises 15%-25% of pediatric HCC, the corresponding figure in adult population is 5% with a relatively better prognosis[8,15,19]. There are diverse genetic pathways and mutations for carcinogenesis in adults with HCC - the common ones being Wnt/beta-catenin (CTNNB1 and AXIN1 genes), telomere maintenance (TERT promoter gene), cell-cycle regulator (TP53 and CDKN2A genes), epigenetic modifier (MLL, ARID1A and ARID2 genes), AKT/mTOR and Ras/raf/MAP kinase (FGF 19, PIK3CA and RPS6KA3 genes), stress oxidative (NFE2L2 and KEAP1 genes) and JAK/STAT (IL6ST and JAK1 genes) pathways[20]. Kim et al[21] compared expression of G1 phase regulatory proteins and loss of heterozygosity (LOH) on chromosomal arms 8p, 13q and 17p in children and adults with HBV-HCC. The expression of cyclin D1 was lower and LOH higher at 13q in childhood HCC. The different staging systems used in adult HCC, like Barcelona Clinics on Liver Cancer (BCLC) staging, Milan criteria, University College of San Francisco (UCSF) criteria, Japan Integrated staging score (JIS), Hong Kong Liver Cancer Classification (HKLC) or under-7 criteria, for purpose of staging and stratifying patients who may or may not benefit from LT have not been prospectively applied in children[11]. Small case series have tested the applicability of Milan criteria in pediatric HCC and found that in contrast to adults LT offers better cure in children falling beyond Milan criteria[14,18,22,23]. The reasons may be related to different biological behavior of the tumor and less aggressive nature of the disease. The survival of children with HCC has improved dramatically over last 3 decades with comparable or even better survival rates than adults with similar severity of disease[13,18,22-26]. This is primarily due to early diagnosis, improvement in surveillance and most importantly, advancement in surgical techniques. Lastly, in contrast to adult HCC which is chemo-resistant and radio-resistant, a proportion (39%) of pediatric HCC respond to chemotherapy[27].
EPIDEMIOLOGY OF PEDIATRIC HCC
Geographical variation in the etiological predisposition for pediatric HCC is largely determined by the prevalence of HBV infection. In regions with high endemicity of HBV, most of the pediatric HCC cases are secondary to perinatally acquired HBV, while in the low endemic regions, tyrosinemia and other metabolic disorders are common causes[7,10,12,18,22,27-31] (Tables 1 and 2).
Table 1.
Clinical characteristic of children with hepatocellular carcinoma in series from East
| Study → Ref. (yr) | Moore et al[7] (2004) | Wang et al[38] (2017) | Chen et al[10,29] (1988, 1998) | Hsu et al[28] (1987) | Zhang et al[12] (2013) | Arikan et al[13] (2006) | Palaniappan et al[26] (2016) |
| Region | South Africa | China | Taiwan | Taiwan | China | Turkey | India |
| No. of subjects | 68 | 65 | 44, 55 | 51 | 45 | 13 (Incidental diagnosis in 3) | 12 (8 diagnosed in explant livers) |
| Males (%) | 68 (78 in HBV subgroup) | 80 | Males dominated | 77 | 93 | 46 | 75 |
| Age (yr) | Mean 10.5 | Median 17 (8-20) | - | Mean 11 (4-15) | Mean 13.5 (3-18) | 6.4 ± 4.8 | Median 5.9 (1.6-15.4) |
| Age-group (n or %) | 91% > 6 yr of age | - | - | 39% < 9 yr of age | - | - | < 5 yr→3 5-10 yr→7 > 10 yr→2 |
| Size (cm) | - | 10.2 ± 4.1 | - | - | > 5 cm in 73% > 10 cm in 67% | Median 3.4 (0.5-8) | 22.5 (2-40) |
| Multifocality | - | 62% | 62% | - | 56% Satellite lesions in 18% | 92% Median number of nodules 3 (1-10) | 42% Median number of nodules 1 (1-5) |
| Etiology | 67% HBV | 82% HBV | 100% HBV | 100% HBV | 71% HBV | 6 Tyr 6 HBV 1 GSD1 | 5 Tyr 2 BA 1 BSEP 1 MDR3 1 NSPILBD 1 PSC 1 Caroli’s |
| Histology (n or %) | HCC 87% FLHCC 13% | - | - | - | - | Well diff 2 Mod diff 4 Poorly diff 6 | Well diff 8 Mod diff 4 (High grade dysplasia or nodules in adjoining liver in 50%) |
| Cirrhosis | - | - | 71%, 68% | 74% (95%) in < 9 yr of age | 47% Child A/B/C 32/8/5 | 100% Mean CTP 11.1 ± 3.1 and PELD 19.7 ± 2.4 (1-44) | - |
| AFP | High in 69% | > 25 ng/mL in 91% | - | - | 93999 ± 31228 ng/mL High in 87% | 8000-250000 ng/dL High in 92% | Median AFP 7.3(1.2-28000) IU/L High AFP (> 100 IU/L) in 42% |
| Extent or stage (n or %) | - | TNM Stage III/IV in 74% | - | Advance stage in 98% | - | TNM stage I (20%), IV (80%) Advance disease in 92% | - |
| Vascular invasion | - | 31% | 42% PV 17% IVC | - | 47% | 54% | 17% |
| Metastases | - | 17% | 29% | - | 24% | 0 | - |
| Resectability | - | 40% | < 10%, 18% | 13% | 27% | NA | NA |
| Outside Milan | NA | NA | NA | NA | NA | NA | NA |
AFP: Alpha-fetoprotein; BA: Biliary atresia; BSEP: Bile salt export pump deficiency (PFIC2); diff: Differentiated; FLHCC: Fibrolamellar variant of HCC; GSD: Glycogen storage disease; HBV: Hepatitis-B virus; IVC: Inferior vena cava; LT: Liver transplantation; MDR3: Multidrug resistance protein3 deficiency (PFIC3); NA: Not assessed; NSPILBD: Non-syndromic paucity of bile ducts; PRETEXT: Pretreatment tumor extent evaluation; PSC: Primary sclerosing cholangitis; PV: Portal vein; TNM: Tumor node metastasis stage; Tyr: Tyrosinemia; Mod: Moderately.
Table 2.
Clinical characteristics of children with hepatocellular carcinoma in series from West
| Study→Ref. (yr) | SEER[8,15,30] (2013, 2013, 2014) | POG[16] (2002) | SIOPEL 1[27] (2002) | Lack et al[31] (1983) | Pham et al[17] (2007) | Ismail et al[22] (2009) | Romano et al[18] (2011) | Beaunoyer et al[23] (2007) |
| Region | United States | United States | Europe | United States | United States | Poland | Italy | United States |
| Number of subjects | 238, 80, 218 | 46 | 39 | 32 | 22 | 21 | 10 | 10 |
| Males (%) | 58 | 57 | 72 | 63 | 50 | - | 40 | 60 |
| Age (yr) | 12.9 ± 5.2 | - | Median 12 (4-15) | Mean 9.7 (0.5-21) | 13.1 ± 1.1 (2-18 ) | Median 11.1 (2-18) | Median 1.8 (0.5-7.2) | Median 10.1 (4.4-16.3) |
| Age-group (%) | 0-4 (8-9) 5-9 (14-16) 10-14 (27-29) 15-19 (48-49) | < 1 y (6.5) 1-9 y (37) ≥ 10 y (56.5) | - | - | - | ≤ 2 (5) 2-10 (38) ≥ 10 (57) | < 2 (50) 2-5 (30) ≥ 5 (20) | 3-12 (70) ≥ 13 (30) |
| Size (cm) | 0-5 (21%) 5.1-10 (24%) 10.1-15 (36%) > 15 (19%) | - | Median 14 (7-26) cm | - | 11.8 ± 0.6 (5-25) cm | - | - | Median 5.8 (2-10.5) cm > 5 cm in 60% |
| Multifocality | Satellite lesions 28% | - | 56% (4 diffuse) | - | - | - | 50% (2-10 in No.) | 70% |
| Etiology | - | - | 13 HBV 1 Tyr 1 Biliary | - | - | 4 Tyr 2 HBV 1 HCV 1 A1ATD 1 AIH 1 PFIC 11 no liver ds | 3 BA 3 PFIC2 2 Tyr 1 CC 1 GSD4 | 4 Viral (3HBV) 1 Tyr 1 Alagille’s 1 PFIC 3 No Liver ds |
| Histology (n or %) | HCC NOS 58%-74% FLHCC 24%-41% Clear cell 1%-2% | HCC 78% FLHCC 22% | Epithelial 75% FLHCC 15% Poorly diff or clear cell 10% | HCC 84% FLHCC 16% | - | High grade2 (29%) Low grade (52%) FLHCC (19%) | G1 (1) G2 (6) G3 (3)2 No FLHCC | HCC in 9 FLHCC in 1 |
| Cirrhosis | - | - | 38% | 16% | - | 52% | 100% | 50% |
| AFP | - | ≥ 20 ng/mL in 67% | Median 9677 (1-1400000) ng/mL > 10 ng/mL in 69% | - | - | 1.7-713000 IU/mL | Median 2322 (3-35000) ng/mL | Median 446927 ng/mL |
| Extent or Stage (n or %) | Local 27%-28% Regional 35%-37% Distant 34%-35% | Stage I (8), II (0), III (25), IV (13)1 | PRETEXT I (1), II (14), III (11), IV (13) | - | CCG/POG Stage I (60%), II (11.5%), III (17%), IV (11.5%)1 | PRETEXT I (4), II (7), III (5), IV (5) | PRETEXT I (4), II (1), III (1), IV (4) | TNM Stage I (0), II (3), III (7), IV (0) T2 (3), T3 (6), T4 (1) |
| Vascular invasion | 48% | - | 21% | - | - | 29% | 20% | 30% |
| Metastases | 35% | - | Lung 31% Other 18% | - | 0 | - | - | |
| Resectability | 25% | 22% | 36% | 22% | 67% | 48% | NA (All LT) | NA |
| Outside Milan | - | - | - | - | - | 86% | 60% | 70% |
Staging done as I, complete gross resection with pathologically negative margins; II, gross total resection with microscopic residual disease at margins; III, gross total resection with nodal involvement or tumor spill; and IV, metastatic disease with either complete or incomplete resection or biopsy;
Grade as per Edmondson and Steiner. A1ATD: Alpha-1 antitrypsin deficiency; AFP: Alpha-fetoprotein; AIH: Autoimmune hepatitis; BA: Biliary atresia; CC: Choledochal cyst; CCG: Children’s cancer group; diff: Differentiated; FLHCC: Fibrolamellar variant of HCC; GSD: Glycogen storage disease; HBV: Hepatitis-B virus; HCV: Hepatitis-C virus; LT: Liver transplantation; NA: Not assessed; PFIC: Progressive familial intrahepatic cholestasis; PRETEXT: Pretreatment tumor extent evaluation; POG: Pediatric oncology group; SEER: Surveillance epidemiology and end-results; SIOPEL: Group for epithelial liver tumors of the international society of pediatric oncology; TNM: Tumor node metastasis stage; Tyr: Tyrosinemia.
Age and gender distribution
As per the SEER database conducted over a period of 1973 to 1997 in United States, pediatric HCC constituted 0.3% of all pediatric malignancies and 31% of all primary hepatic malignancies. The overall age-adjusted incidence rate of pediatric HCC was estimated to be 0.41 (0.24-0.65) per 1000000. The rates were slightly higher for males than females (0.45 vs 0.37 per 1000000). Of these, 12.9% were among children below 5 years of age and 34% were between 15 to 19 years of age. Incidence is highest (0.8 per million) among adolescents. This is in contrast to hepatoblastoma where a majority (91.3%) of cases belong to under-5 year age group[5]. In most of the studies, males outnumbered females with a factor ranging from 1.27 to 2.45[6-8,12-18].
Epidemiological studies from the East have shown higher incidence of pediatric HCC as compared to West and this is primarily related to the high prevalence of perinatally acquired HBV infection. In certain areas it was reported as the commonest childhood liver malignancy[6,7]. There is bimodal age distribution of cases with first peak at around 1 year of age, then a decline to trough at 4 years and a second peak at around 12 to 15 years of age[6]. Male preponderance is more marked in the East with a male to female ratio of 2.1-13.3:1[6,7,12]. A South-African study has found a gender ratio-difference of 2.5 between Hepatitis-B surface antigen (HBsAg) positive vs negative cases of pediatric HCC[7].
Racial differences
As per SEER database, whites and non-Hispanics comprise 72% and 82% of pediatric HCC, respectively[8]. Hence, the racial differences are not as pronounced as in adults with HCC.
Time-trend of pediatric HCC
Epidemiological data from 2003 has shown that there is a declining trend in pediatric HCC from 1973 to 1997. This is in contrast to almost double the number of cases of hepatoblastoma[5]. In a subsequent analysis by SEER published in 2013, the overall incidence of pediatric HCC has been estimated to be 0.59 per million per year with a relatively stable incidence over last 4 decades (0.62 in 1970s, 0.43 in 1980s, 0.59 in 1990s and 0.62 in 2000s per million per year)[8]. Contrastingly, data from East has shown an 85% reduction in the incidence of pediatric HCC 20-years following introduction of HBV immunization[32].
Changing epidemiology in the East following hepatitis-B vaccination
There has been a dramatic change in the incidence of HBV related HCC in pediatric age-group following introduction of mass immunization against HBV in the Asia-Pacific region. Chang et al[33] showed that following universal HBV vaccination, the average annual incidence of HCC in children 6-14 years of age declined from 0.70 to 0.36 over a decade. This translated in a corresponding (50%) reduction in mortality due to pediatric HCC in the same age group. Study by Taiwan Childhood Hepatoma Study Group further showed that the vaccination program also resulted in reducing boy-girl incidence ratio for the disease from 4.5 in 1981-1984 to 1.9 in 1990-1996. There was a dramatic reduction in incidence of disease in boys (relative risk 0.72) over this time period. However, for girls there was no decrease in the incidence and with increasing age a rising incidence was observed[34]. Subsequent analysis by the same group of workers showed that vaccination resulted in almost 70% reduction in the risk of developing HCC adjusted to age and gender. Moreover, in the vaccination cohort, the risk of developing HCC was found to be related to incomplete vaccination (odd’s ratio, OR = 4.32), prenatal maternal HBsAg seropositivity (OR = 29.5), prenatal HBeAg seropositivity [with administration of HBV immunoglobulin (OR = 5.13) and without it (OR = 9.43)][32].
PATHOGENIC RISK FACTORS
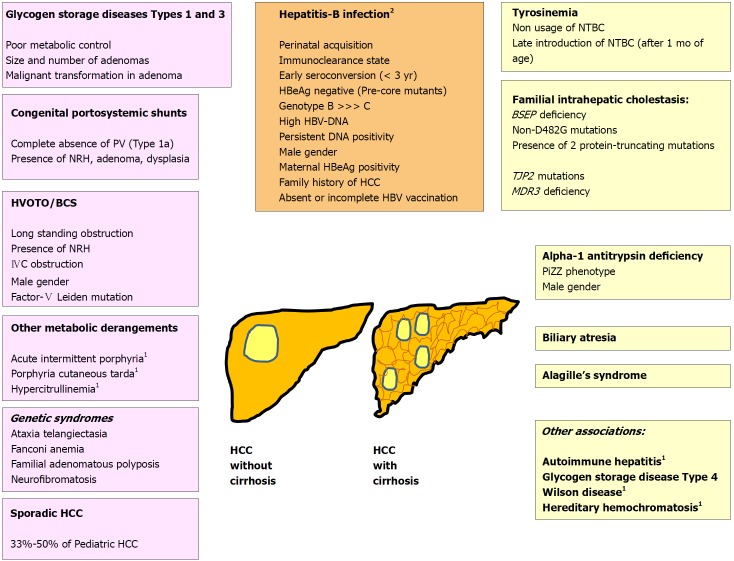
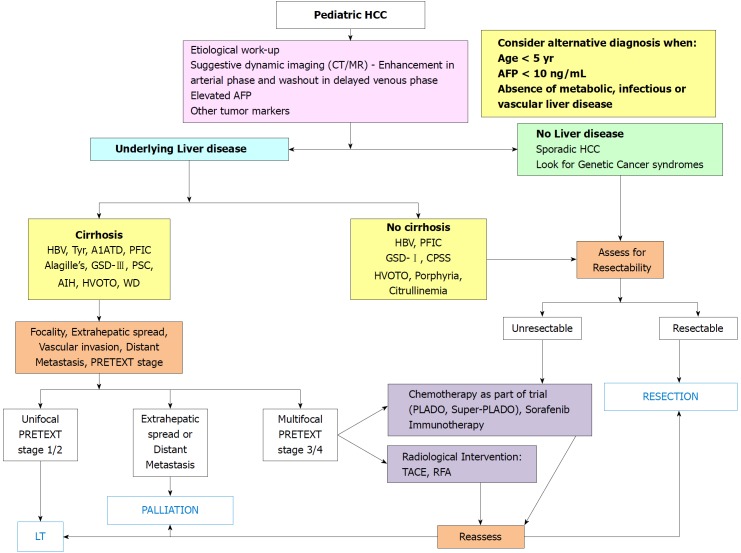
Various pathogenic risk factors of pediatric HCC have been illustrated in Figure 1. Hepatocellular cancer may develop in a cirrhotic or a non-cirrhotic liver, and in the later scenario it may or may not be associated with underlying liver disease. Differentiation on the basis of these two points is important for treatment allocation and outcome as shown in Figure 2.
Figure 1.
Risk factors for pediatric hepatocellular carcinoma. 1Conditions cause HCC in adults, and very rarely in children; 2HBV related HCC may occur in presence or absence of cirrhosis. BCS: Budd-Chiari syndrome; HCC: Hepatocellular carcinoma; HVOTO: Hepatic venous outflow tract obstruction; IVC: Inferior vena cava; NRH: Nodular regenerative hyperplasia; BSEP: Bile salt export pump; HCC: Hepatocellular carcinoma; MDR3: Multidrug resistance protein-3; NTBC: [2-(2-nitro-4-trifluoromethylbenzoyl)-1,3-cyclohexanedione (Nitisinone); PiZZ: Homozygous PiZ phenotype of alpha-1 antitrypsin; PV: Portal vein, TJP: Tight junction protein.
Figure 2.
Algorithmic management of pediatric hepatocellular carcinoma. A1ATD: Alpha-1 antitrypsin deficiency; AFP: Alpha-fetoprotein; AIH: Autoimmune hepatitis; CPSS: Congenital portosystemic shunt; GSD: Glycogen storage disorder; HBV: Hepatitis-B virus; HVOTO: Hepatic venous outflow tract obstruction; LT: Liver transplantation; PFIC: Progressive familial intrahepatic cholestasis; PLADO: Cisplatin + Doxorubicin; PRETEXT: Pretreatment tumor extent evaluation; PSC: Primary sclerosing cholangitis; RFA: Radiofrequency ablation; Super-PLADO: Cisplatin, Carboplatin and Doxorubicin; TACE: Transarterial chemo-embolization; Tyr: Tyrosinemia; WD: Wilson disease.
Hepatitis-B
Hepatitis-B infection is the commonest cause of HCC in adults who live in regions with high endemicity like Southeast Asia and sub-Saharan Africa. HBV constitutes 65% of HCC cases in adults from China and Far East as compared to < 20% from United States. Chronic carriers of HBV have upto 30-fold increased risk of development of HCC[11]. Pediatric HCC in the setting of HBV infection usually develops with perinatally acquired infection and is more common in males with genotype B[31,35]. Gender disparity is universal for all types of HCC and is related to the protection against the tumor by estrogen via a complex pathway involving hepatocyte nuclear factor-4[36]. Family studies from Far-East have confirmed that early infection with HBV, particularly through HBsAg positive mother, leads to persistent chronic infection in children, subsequently leading to development of chronic hepatitis, post-necrotic cirrhosis and HCC[36,37]. Majority of tumors are multi-centric, although cirrhosis may be absent in 26%-53% of cases[12,38] (Figures 3 and 4). Pediatric HCC secondary to HBV differs from non-HBV cases with regard to male predominance (68%-93% vs 50%), older age of presentation (14.5 years vs 10.9 years), presence of cirrhosis (56% vs 23%) and portal vein invasion (56% vs 23%), but there is no difference with respect to tumor size, number of lesions or metastases[6-8,12-16,30]. Thus, HBV related HCC represents more aggressive and advance disease, and complements its adult counterpart. An old study from Taiwan on HBV related pediatric HCC showed that the presence of cirrhosis is more common in younger children below 9 years of age (95% vs 58%). Hepatitis-B early antigen (HBeAg) in serum and hepatitis-B core antigen in the liver tissue were detected in 18% and 11% of cases, respectively. This coupled with high frequency of cirrhosis especially in the younger children indicated that early seroconversion from HBeAg to anti-HBe in association with severe liver injury plays an important role in development of HCC[28]. In a large cohort of chronic hepatitis-B children from India, HCC was detected in 4.3% at an age between 9 to 14 years with elevated transaminases, however 80% didn’t have cirrhosis[39].
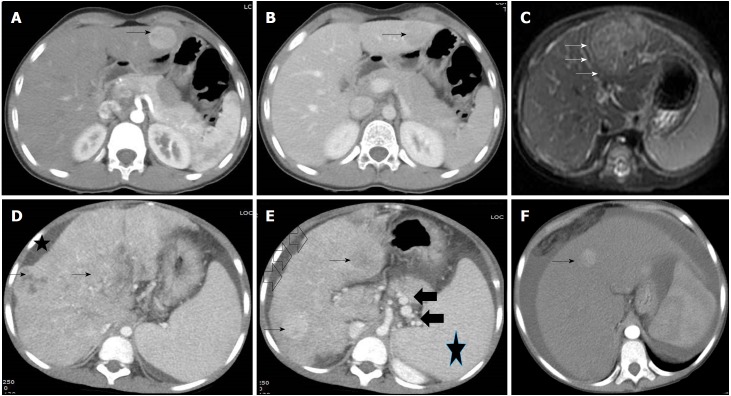
Figure 3.
Radiological images in children with hepatocellular carcinoma. A and B: 14 and half years old boy with chronic hepatitis-B (Immunoclearance phase), HBeAg+, DNA 5 log, AFP = 3 ng/mL (normal), with a segment 3 lesion (2.5 cm × 2.4 cm), BCLC stage A. Contrast-enhanced CT (CECT) image showing enhancement of the lesion (arrow) in the arterial phase (A) and washout in delayed venous phase (B) in a background of non-cirrhotic liver; C: 10 mo old boy with infantile cholestasis, bile salt export pump (BSEP) deficiency on immunostaining, incidentally found to have lesion in segment II and IVa abutting the capsule, BCLC stage B. The lesion was T2 hyperintense with enhancement in the arterial phase (arrows) and washout in the delayed phase; D and E: 8-1/2 yr old boy with decompensated chronic liver disease, chronic hepatitis-B (Immunoclearance phase), HBeAg+, DNA 7 log, AFP = 250000 ng/mL, with multifocal HCC, BCLC stage D, Child-Pugh C. Contrast-enhanced CT (CECT) image showing heterogenous lesion (arrows, D and E) involving whole of the right lobe with enhancement in the arterial phase in few lesions (arrows, E) in a background of cirrhotic liver with nodular margins (open black arrows, E), ascites (star, D) and collaterals (black solid arrows, E) and splenomegaly (star, E); F: 14 yr old girl with hepatic venous outflow tract obstruction, ascites, portal hypertension, incidentally found to have a lesion in segment 4 (1 cm × 1 cm), BCLC stage D, Child-Pugh C. Contrast-enhanced CT (CECT) image showing enhancement of lesion in the arterial phase (arrow).
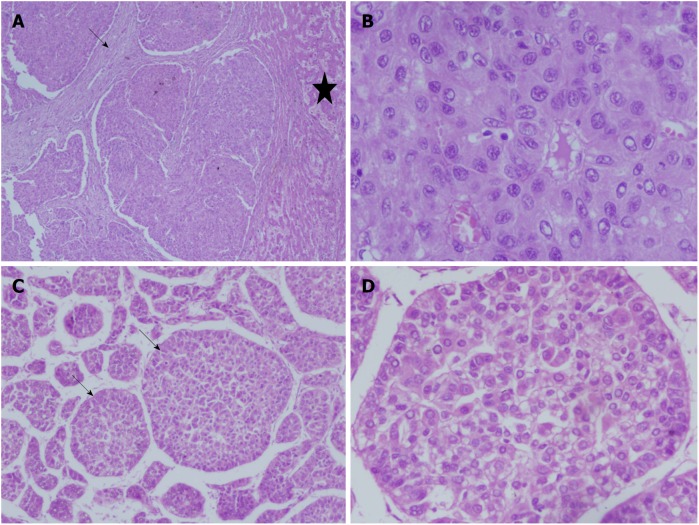
Figure 4.
Liver histology images in children with hepatocellular carcinoma. A and B: 15 yr old boy with HBeAg negative chronic hepatitis-B, DNA 4 log, precore mutant, AFP = 150000 ng/mL, with a large HCC in right lobe, with portal vein thrombosis, BCLC stage C, Child Pugh A, underwent right hepatectomy. Low power view showing tumor nodules separated by fibrous bands (arrow), adjacent non-malignant parenchyma (star) (100 ×, HE stain) (A). High power view showing malignant nuclear details with prominent nucleoli and eoisnophilic cytoplasm (400 ×, HE stain) (B); C and D: 14 and half years old boy with chronic hepatitis-B (Immunoclearance phase), HBeAg+, DNA 5 log, AFP = 3 ng/mL (normal), with a segment 3 lesion (2.5 cm × 2.4 cm), BCLC stage A, underwent non-anatomic resection. Low power view of tumor showing broadened trabeculae (arrows) (100 ×, HE stain) (C). Trabeculae with malignant cytological features - nuclear atypia and anisocytosis (200 ×, HE stain) (D).
Numerous risk factors for development of HBV related HCC have been studied in adults. Concomitant liver insults like hepatitis-C, human immunodeficiency virus, alcohol intake, smoking, non-alcoholic fatty liver disease, diabetes, ingestion of aflatoxin from Aspergillus flavum are common in adults but have not been studied in children[11]. Similarly, numerous genetic associations are known in adults with HBV-HCC, namely loss of heterozygosity on the KIF1b locus, deletion or chromosomal loss of DLC1 (Deleted in Liver Cancer 1) locus, mutations in cytotoxic T-lymphocyte antigen 4 (CTLA-4) gene, promoter region of the Minichromosome maintenance protein-7 (MCM7) gene and enhancer II (EnhII), basal core promoter (BCP) and precore regions of HBV[40]. It has been shown that Pre-S mutations, C1653T, T1753V, and A1762T/G1764A are associated with an increased risk of HCC[41]. A recent study from China on mother-cord-infant pairs has shown that the HCC-risk mutations were present in the mothers’ peripheral blood and cord blood but mostly absent in peripheral blood of 7-mo-old infants. Further, in 56 mother-child pairs with documented mother to child transmission of infection, it was shown that HCC mutations evolved over first 15 years of age - Pre-S deletion in genotype B2; C1653T, G2946A/C, C1T/A, A7C, and pre-S1 start codon mutation in genotype C2; and A1762T/G1764A and G1896A in both the genotypes, the mutations consecutively increased in frequencies with increasing age[42].
Tyrosinemia
Tyrosinemia type I or hepatorenal tyrosinemia is caused by deficiency of enzyme fumaryl acetoacetate hydrolase (FAH). Due to blockage in the last step of the tyrosine degradation pathway, there is accumulation of toxic metabolites - maleylacetoacetate, fumarylacetoacetate (FAA), and succinylacetone (SA) - which lead to hepatic and renal manifestations of the disease and carcinogenesis. The incidence of HCC in tyrosinemia is reported to be 14%-75%, the prevalence increases with age of the child[43,44]. Nitisinone [2-(2-nitro-4-trifluoromethylbenzoyl)-1,3-cyclohexanedione, (NTBC)] blocks the enzyme parahydroxyphenylpyruvic acid dioxygenase, which is the second step in the tyrosine degradation pathway, and thus prevents accumulation of FAA and SA. Usage of NTBC along with low tyrosine diet leads to survival rates greater than 90% in these children. Further, if NTBC is started early (within 30 d of birth), there is reduction in incidence of HCC from 37% to 1%, and need of LT from 71% to 26%[43-45]. However, animal models have shown that NTBC treatment fails to normalize the tyrosinemia-induced alterations in expression of transcripts encoding proteins involved in protein turnover, signal transduction, and cell growth and proliferation[46]. One of the studies on 16 children who underwent LT at a median age of 16 mo has shown the presence of HCC in 12 (75%) liver explants - 7 were multifocal, 3 with microvascular invasion, and all were well differentiated. There was 86% tumor-free survival at a median of 6.6 years[47]. Contrastingly, report from Iran has shown that HCC was present in 5 (23%) of the 22 explants, the diagnosis was confirmed before LT in 2 children[48].
Progressive familial intrahepatic cholestasis
Among the list of Progressive familial intrahepatic cholestasis (PFIC) disorders, Bile salt export pump (BSEP) deficiency or PFIC type-2 children are especially predisposed at a young age to develop HCC (Figure 3). There is poor excretion of bile salts through the canalicular membrane leading to constant exposure of hepatocytes to bile salts with chronic inflammation and carcinogenesis. Studies from United States and Europe showed that HCC occurs in 5%-15% of children with BSEP deficiency at a young age (13 to 28 mo)[49-52]. Children with D482G mutations have less severe disease and portal hypertension, while HCC is common in those with non-D482G mutations[51]. From the largest cohort of 128 European children, single-strand conformation polymorphism analysis and sequencing of ABCB11 gene identified high risk of HCC (38% vs 10%) in children with presence of 2 protein-truncating mutations[52]. Exome sequencing of the genomes of humans affected by BSEP and of Mdr2 knock-out mice revealed that a very few somatic mutations accumulated over time in the cancer genes. This stands in contrast to adults with HCC as well as other malignancies where a number of mutations accumulate over a period of time. Further, in BSEP individuals and animals, there is massive gene amplification that affected components of signal transduction pathways, such as the ErbB, the PI3K/Akt and the mitogen-activated protein kinase (MAPK) signalling pathways and in particular, activators of c-Jun-N terminal kinases (JNK)[53]. Another study has provided further pathophysiologic insights into BSEP mediated HCC. It showed that BSEP expression is severely diminished in HCC patients associated with alteration of farsenoid-X receptor (regulatory nuclear receptors) with increase in (FXR-α1/FXR-α2) ratio, the later is induced by inflammation and may be reversible[54].
HCC has also been described in a new variant of PFIC with severe cholestasis - TJP2 (tight junction protein 2) deficiency. Protein truncating mutations in TJP2 gene cause failure of protein localization and disruption of tight junction structure leading to severe cholestatic liver disease. In presence of these mutations, Claudin (CLDN1) fails to localise normally to cholangiocyte borders and biliary canaliculus margins, despite normal protein levels. In the unusually hostile environment of the canalicular and cholangiocytic membranes, exposure to high concentrations of detergent bile acids due to TJP2 deficiency lead to hepatobiliary disintegrity, producing severe liver injury and HCC[55,56]. Finally, in multidrug resistance protein-3 (MDR3) deficiency (PFIC type 3), HCC has been reported but is less common than BSEP deficiency[57].
Glycogen storage disorders
In Glycogen storage disorders, HCC develops on a soil of adenoma (adenoma-carcinoma sequence). Adenomas are common in Glycogen storage disorder (GSD) type I (glucose-6-phosphatase deficiency) with a frequency of 16%-75%. Most of these are seen in the second and third decade with a mean age between 11 to 19 years. In comparison to adenomas in general population, adenomas of GSD-Ia children are more in number, bilobar in distribution, and without any gender predisposition. There is regression in size and number of adenomas with good metabolic control. Malignant transformation in such adenomas occurs rarely, and in view of rarity of the event as well as the disease, it is difficult to estimate the overall risk[58-61]. However, the risk is related to size, number and duration of adenomas, and their recurrence[60]. There is no effective biomarker as alpha-fetoprotein (AFP) and carcinoembryonic antigen levels are often normal in this setting. In a genetic study, it has been shown that chromosomal aberrations are present in 60% of GSD-Ia adenomas which is almost comparable to general population adenomas, but simultaneous gain of chromosome 6p and loss of 6q were only seen in GSD-Ia adenomas. Moreover, these adenomas also showed reduced expression of insulin-like growth factor-2 receptor (IGF2R) and large tumour suppressor kinase-1 (LATS1) candidate tumor suppressor genes at 6q in more than 50%. This indicates a possible role of chromosome 6q in malignant transformation of such adenomas[62].
Type III GSD (debrancher enzyme deficiency, or Forbes disease, or limit dextrinosis) differs from type I in that the liver enzymes are elevated with hepatocellular injury, and evidence of ballooning degeneration and fibrosis. Adenomas are seen in 4%-25% of patients. Malignant transformation takes place less often, but is almost always in the background of cirrhosis[63,64]. Liver cancer is rarely seen in Type 4 GSD (brancher enzyme deficiency, or Andersen’s disease, or Amylopectinosis), but has never been reported in types 6 and 9 diseases[18].
Alpha-1 antitrypsin deficiency
Individuals with homozygous (PiZZ) alpha-1-antitrypsin deficiency (A1ATD) are at an increased risk for liver damage, cirrhosis and HCC. Liver cancer is rare in the pediatric age group but has been reported[22]. In a recent systemic review, the prevalence of HCC in adults with A1ATD is 1.3% with a low yearly cumulative rate of HCC than other causes of cirrhosis (0.88% per year vs 2.7% for hepatitis-C cirrhosis)[65,66]. However, autopsy data in adults has shown the prevalence of HCC of 16%[67]. Animal models of A1ATD have shown that approximately 69% of the PiZZ mice develop tumors by 16-19 mo of age and HCC was present without evidence of pre-cancerous lesions or benign adenoma. There was upregulation of Cyclin-D gene, and elevation of c-Fos and melanoma cell adhesion molecule (MCAM), all three factors involved in cell proliferation, tumorigenesis and metastasis[68]. The liver injury is related to increased oxidative stress with an upregulation of redox- regulating genes and reduction in levels of antioxidant enzymes[69].
Alagille’s syndrome
Alagille’s syndrome is a multisystem disorder characterized by bile ductal paucity and chronic cholestasis. Mutations/deletions in two genes are known to cause Alagille’s syndrome: JAGGED1 (encoding the Notch signaling pathway ligand JAGGED1) in 89% and NOTCH2 (encoding one of the Notch receptors) in a small minority. Carcinogenesis in Alagille’s syndrome is rare but reported in children and adults[23,70,71]. HCC in Alagille’s syndrome is seen in first or second decade in a background of cirrhosis with extensive pruritus[71]. Constitutive intracellular domain of Notch2 signalling in the liver leads to up-regulation of pro-proliferative genes and proliferation of hepatocytes and biliary epithelial cells, and stimulates diethylnitrosamine induced HCC formation in mice[72]. Recently, yes associated protein (YAP), which is a transcriptional co-activator and is responsible to make cancer cells proliferate in an anchorage independent fashion and become apoptosis resistant, has been shown to act by upregulating JAGGED-1 and activation of NOTCH pathway in mouse models and humans with HCC, colorectal and pancreatic cancers[73]. This may have putative role in Alagille’s syndrome related HCC, and need to be studied.
Congenital portosystemic shunts
Nodular transformation in livers of children with congenital portosystemic shunts (CPSS) is related to differential vascular supply[74]. Such nodules may be benign or malignant. Two largest reviews on CPSS and largest series from France have shown that extra-hepatic CPSS (59%) is slightly more common than intra-hepatic CPSS (41%)[74-76]. The common communications are patent ductus venosus, portal vein to inferior vena cava (IVC), or left portal venous system to IVC[74]. Liver masses have been described in both extra-hepatic (35%) and intra-hepatic (13%) types at a median age of 8 years, however the malignant tumors (hepatoblastoma, HCC and sarcoma) are specifically described in extrahepatic variety. As per available literature, HCC is seen in 2.5% of CPSS cases[76]. Shunt occlusion leads to reduction in size of benign tumors like focal nodular hyperplasia and hepatic adenoma, however for malignant tumors, resection and shunt occlusion or LT is indicated[74].
Biliary atresia: Study from King’s College London has shown that the prevalence of HCC in biliary atresia is 1.3% (5 out of 387). All except one were below 5 years of age (range 1.1-4.9 years)[77]. On the other hand, biliary atresia constitutes 0-30% among all causes of pediatric HCC, particularly from the West, however the figures may indicate a referral and surveillance bias[18,22,23,26,27]. Cancer in biliary atresia develops on a soil of biliary cirrhosis with ongoing chronic necroinflammation and destructive cholangitis[77,78].
Budd-Chiari syndrome: HCC is a known complication of Budd-Chiari syndrome in adults with a 5-year cumulative incidence of 4%. It commonly develops in a liver with nodular regenerative hyperplasia, and is more commonly associated with male gender, factor-V Leiden mutation and inferior vena cava obstruction[79]. We have described HCC in a 14 years old girl with Budd-Chiari syndrome and Celiac disease[80] (Figure 3).
Other liver disorders: Autoimmune hepatitis (AIH) and Wilson disease (WD) are two commonest chronic liver diseases in older children[81]. From a systematic review and meta-analysis on adults with AIH, the risk of HCC is 3.06 per 1000 patient-years, slightly lower than that with other causes of cirrhosis in adults like hepatitis-B, C, or primary biliary cholangitis[82]. The risk of HCC is related to presence of cirrhosis at the time of diagnosis of AIH (OR = 4.08) and abnormal transaminases at final observation (OR = 3.66)[82,83]. Although incidence of HCC in children is rare, but as AIH is a common pediatric liver condition so it is important to be aware of the carcinogenesis risk in the long term. Copper accumulation in WD is said to be protective against tumorigenesis. The estimated annual risk of HCC from a Dutch study on 140 adults with WD followed up over 15 years was calculated to be 0.09%[84]. From another multicenter European study of 1186 WD patients, the prevalence of HCC was found to be 0.67%[85]. Hence, the risk is significantly low in WD to advocate regular HCC surveillance. Non-alcoholic fatty liver disease, type 2 diabetes, hereditary hemochromatosis and porphyria (acute intermittent and porphyria cutanea tarda) carry high risk of development of HCC in adults, but not in children[86].
Genetic cancer syndromes and sporadic HCC
Certain genetic cancer syndromes confer high risk of development of HCC in the absence of a metabolic, infectious or vascular disease of liver (Figure 1). Sporadic HCC developing in the absence of a known or unknown liver disease or genetic cancer predisposition is seen in 0-62% of children with HCC, the proportion is high in areas with low endemicity of HBV infection[10,12,13,18,22,23,27-29,31].
PATHOLOGY
Histologically, most of the pediatric HCCs resemble the adult type. The common proliferation patterns are solid, trabecular, acinar or scirrhous, and occasionally clear cell or steatotic change[47,87]. According to the SEER database, the most common histological subtype of pediatric HCC is non-fibrolamellar HCC constituting 73% of cases, followed by fibrolamellar HCC in 25% and clear cell carcinoma in 2% of cases. These histological subtypes do not differ with regard to gender, race, ethnicity and tumor stage at presentation[8]. Various grading systems including Edmondson and Steiner grading have been described[13,14,18,22,47]. Tumor grades I and II or low grade tumors (28%) refer to neoplastic cells with small nuclei which are slightly enlarged, hyperchromatic, and have single mitotic figure. While, high grade tumors (III and IV) comprise cells with large, hyperchromatic pleomorphic nuclei with numerous mitotic figures and sometimes anaplastic foci[22] (Figure 4). An elegant study from London described pathological details of 26 HCC nodules in 12 children - morphology was solid in 62%, trabecular in 35% (two-thirds with thick trabeculae) and scirrhous in 4%. HCC in children with tyrosinemia are more often well differentiated, have grade I morphology, and are solid or trabecular with diffuse clear cell change[87]. Premalignant dysplastic foci are commonly found in adjoining areas of tyrosinemia livers[47,87]. HCC in biliary atresia have moderately pleomorphic nuclei with eosinophilic cytoplasm arranged in a trabecular pattern. In BSEP deficiency, HCCs have varied morphology and proliferation pattern. On immunohistochemistry, epithelial cell adhesion molecule (EpCAM), cytokeratin (CK)-19 and Glypican-3 have been found to be more commonly expressed in pediatric HCC in contrast to adult HCC. In contrast to hepatoblastoma, pediatric HCC livers less frequently expressed beta-catenin and more frequently expressed p53. Further in contrast to fibrolamellar HCC, there was less expression of CK-7, which is a marker of biliary proliferation[87].
CLINICAL FEATURES
The common symptoms of pediatric HCC are abdominal mass and pain. Children with advance disease often have cachexia and jaundice. Other signs and symptoms of decompensated end-stage liver disease and portal hypertension (ascites, variceal bleed, encephalopathy, spider naevi, clubbing) are present in children with underlying cirrhosis. Upto one-third of pediatric HCCs are detected incidentally on imaging[13,14]. Liver is usually firm to hard in consistency with irregular margins. Nodular surface may be appreciable. Splenomegaly and ascites are related to the degree of portal hypertension (Tables 1 and 2).
DIAGNOSIS
The diagnosis of HCC in a child with cirrhosis or a predisposing liver disease is usually presumed on basis of elevated AFP levels and abnormal nodule (space occupying lesion) on ultrasound.
Imaging
Ultrasound typically shows presence of a heterogenous hyperechoic mass of variable size with increased vascularity[2]. For further characterization of the mass, a dynamic imaging, either contrast-enhanced computerized tomography (CECT) or magnetic resonance (CEMR) imaging is needed. Typical characteristics of HCC on dynamic imaging are hyper-enhancement in the arterial phase (10-20 s) and washout in the portal venous (60-80 s) and delayed venous (3-5 min) phases. The “enhancement” is because of the intranodular arterial supply of HCC, while “washout” is related to early venous drainage of the contrast, progressive enhancement of background cirrhotic liver, reduced intra-nodular portal venous drainage, tumoral hypercellularity with corresponding reduction in extracellular volume, and intrinsic hypoattenuation/hypointensity[88] (Figure 1). From the recent adult systematic review and meta-analysis, it was found that CEMR has higher sensitivity (82% vs 66%) and lower negative likelihood ratio (0.20 vs 0.37) over CECT for the diagnosis of HCC[89]. It has been proposed that in adults with non-typical characteristics on imaging, combined interpretation of dynamic and hepatobiliary phase of Gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid (Gd-EOB-DTPA)-enhanced MRI with diffusion-weighted imaging (DWI) can improve the diagnostic yield of HCC[90]. Although MR avoids radiation hazard to young children, in view of technical complexity, expertise, availability, susceptibility to motion artifacts, need for intubation and poor image quality in presence of ascites, CT is mostly preferred to look for tumor extent, vascular invasion, resectability and metastases[91]. Contrast-enhanced ultrasound (CEUS) has been shown to be promising in children with an advantage of minimizing the exposure to ionizing radiation. In a study on 44 children with focal liver lesions, agreement between CEUS and other imaging modality (CT or MR) was seen in 85%, with the specificity and negative predictive value of CEUS of 98% and 100%, respectively[92]. Sonovue® (sulfur hexafluoride with phospholipid shell), used as contrast for CEUS, has been tested for other indications in pediatric age-group and is found to be extremely safe[93].
Tumor markers
Serum AFP elevation, not very sensitive, but has a fairly good specificity for detection of HCC in at risk population with liver disease. In adults, it also has a role in prognostication and surveillance[91]. Because of the low incidence of HCC in pediatric age-group and the fact that AFP levels are elevated in presence of regeneration, the exact role of surveillance with AFP may be limited. Nevertheless, the levels are mostly high (67%-92%), as high up to 1400000 ng/mL[7,12-14,16,18,22,26,27] (Tables 1 and 2). Caution should be taken in children with tyrosinemia with very high AFP values, where a serial change from the baseline should be considered more informative. Other tumor markers like fucosylated AFP or Lens culinaris agglutinin-reactive fraction of AFP (AFP-L3), des-gamma-carboxy prothrombin (DCP) or protein adducts in absence of vitamin-K absence (PIVKA-II), glypican-3, Golgi protein-73, hepatocyte growth factor, insulin growth factor 1 and transforming growth factor-β1 have not been prospectively studied in children[90].
Cytology/biopsy
As per the adult literature on HCC, American association for study of liver diseases (AASLD) does not suggest biopsy in patients with cirrhosis with a lesion radiologically suggestive of HCC. For lesions which are indeterminate on imaging, repeating a different imaging, or with another contrast, or biopsy is suggested to confirm the diagnosis[91]. APASL consensus suggests biopsy when the nodule is non-hypervascular, or hypervascular without washout with a size of ≥ 1 cm[90]. No such recommendations exist for pediatric age-group. However, we suggest that in children without cirrhosis, histological evaluation should be done. While in those without cirrhosis, diagnosis should be based on suggestive imaging and high AFP.
DIFFERENTIAL DIAGNOSIS
Hepatoblastoma is an important differential of HCC especially in children younger than 5 years of age. Predisposing factors like low birth weight, maternal pre-eclampsia, parental smoking, and certain syndromes like hemihypertrophy (Beckwith-Weidmann syndrome), familial adenomatous polyposis, Li-Fraumeni syndrome, Trisomy 18 and Simpson Golabi-Behmel syndrome favor hepatoblastoma. Imaging features and AFP may not help to differentiate from HCC. Presence of calcification, cystic areas or necrosis may suggest hepatoblastoma. Evidence of embryonal-type epithelial or mesenchymal elements on histopathology is almost diagnostic of hepatoblastoma. However in the presence of trabecular architecture or well differentiated fetal epithelial cells, a definite diagnosis may be difficult[1,2]. A term “hepatocellular neoplasm not otherwise specified” has been coined for such tumors[94]. In older children, other benign and malignant lesions like focal nodular hyperplasia, adenoma and undifferentiated embryonal sarcoma should be considered in the differential of HCC[1].
MANAGEMENT
Staging
Most of the groups working on pediatric liver tumors like Group for Epithelial liver tumors of the International Society of Pediatric Oncology (SIOPEL), Pediatric Oncology group (POG), Children’s Cancer Group (CCG) and Japanese study group for Pediatric Liver tumor (JPLT) have been using pre-treatment extent of tumor (PRETEXT) staging system. This system has been recently updated by the Children’s Hepatic tumors International Collaboration (CHIC), and has been found to be a powerful predictor of overall survival of children with hepatoblastoma and HCC. In contrast to adult HCC, where size and number based classifications are more popular for purpose of decision about resection, transplantation or palliation, the PRETEXT system is based on determining number of contiguous tumor-free liver sections - left lateral, left medial, right anterior and right posterior. These sections are divided from right to left by (1) right hepatic vein, (2) Cantlie’s line, and (3) a plane extending along hepatic fissure and umbilical portion of left portal vein. The stage is determined by calculating the number of contiguous sections that have to be resected to completely remove the tumor. The stage further includes annotation factors like V (hepatic venous or inferior vena cava involvement), P (portal vein involvement), E (extrahepatic disease contiguous with main liver tumor), F (multifocality), R (tumor rupture), C (caudate lobe involvement), N (lymph node metastases) and M (distant metastases)[95]. Application of Milan criteria and BCLC staging system in children is limited[14,18,22,23].
Treatment modalities and outcome
Table 3 summarizes various treatment modalities and outcome of children with HCC[6-8,10,12-18,23-31,38,96-101]. The main factors considered for decision making in adults with HCC are (1) Liver functional status - ascites, albumin, bilirubin, alkaline phosphatase, portal vein thrombosis, Child-Turcotte-Pugh (CTP) score; (2) tumor factors - size and number of nodules, extent, vascular invasion, presence of metastases, TNM staging, AFP levels; (3) portal hypertension - presence of varices, hepatic venous pressure gradient (HVPG); (4) general status of patient - performance status, symptoms[11,90,91,102,103]. Most of the clinical experience obtained from treatment of hepatoblastoma has been applied for treatment of HCC in children. The best option for non-metastatic HCC tumors is complete surgical removal either by resection or LT. Resection offers good cure rates, however only 27% (range 10%-67%) of tumors are resectable. Over a period of 4 decades there has been a dramatic improvement in the 5-year survival rates of children with HCC from 4%-10% to 56%-80% - the change is primarily related to increased surveillance and improvement in surgical techniques, particularly LT[7,8,10,12,28,29,35,96-98]. An algorithmic approach for management of these children is presented in Figure 2. All children with non-metastatic HCC should be assessed for resection or LT to achieve optimal outcomes. Systemic neoadjuvant chemotherapy can be used in children awaiting LT to serve as a “bridge” to LT and prevent disease progression. Interventional radiology techniques using transarterial locoregional chemotherapy can be tried in older children with large unresectable tumors and to control tumor burden as a “bridge” to resection or LT.
Table 3.
Changing outcome of children with hepatocellular carcinoma over last 4 decades
| Study (yr) Ref. | Number of patients | Factors governing outcome | Intervention (s) done | Survival |
| Conservative treatment (Observation and resection) | ||||
| Lack et al[31] (1983) | 32 (5FLHCC) | Higher resectability and overall survival with FLHCC | Observation Resection | 5 yr 7% MST of HCC 4.2 mo and FLHCC 28.5 mo |
| Wu et al[96] (1987) | 20 | - | - | 5 yr 0; MST 4.7 mo |
| Hsu et al[28] (1987) | 51 | Early HBeAg seroconversion with severe liver injury predispose to HCC | Observation Resection | 1 yr 10.5% |
| Chen et al[29] (1988) | 44 | No difference in survival with chemotherapy | Observation Resection | 5 yr 7% |
| Ni et al[97] (1991) | 71 | Favorable prognosis with resectability and absence of icterus | Observation Resection | 1 yr 10%, 5 yr 4% |
| Lee et al[6] (1998) | 28 | - | - | 5 yr 17% |
| Hsiao et al[98] (2009) | 13 | - | - | DFS 30% |
| Allan et al[8] (2014) SEER database | 218 | Reduced mortality associated with resectability (OR = 0.18), non-Hispanic (OR = 0.52), local disease (OR = 0.46) | - | 5 yr 24%, 10 yr 23%, 20 yr 8% |
| Mixed treatments (chemotherapy/TACE/liver transplantation) | ||||
| Tagge et al[99] (1992) | 21 | Total hepatectomy and LT improved survival in those with unresectable disease | Surgery in 15 (6 PH, 7 LT, 2 Exenteration and MOT) Pre-operative CT in 2 Observation in 6 | 1 yr 29% |
| Chen et al[10] (1998) | 55 | Good outcome with resection, poor with unsatisfactory resection & metastases Distant metastases carries worst prognosis | Resection CT Observation | MST with resection 23 mo, CT 3 mo and no treatment 2 mo |
| Moore et al[7] (2004) | 68 | - | Resection ± CT TACE Observation | > 5y 11% MST 4 mo |
| Pham et al[17] (2007) | 22 | - | Surgery ± CT | OS 5 yr 30% MST 23 mo |
| Zhang et al[12] (2013) | 45 | Low overall survival with metastases & non-resectability, but unrelated to HBsAg positivity Large tumor size, early metastasis, bilateral involvement, and PV invasion precluded resection | Resection TACE Observation | 1 yr 34%, 3 yr 4%, 5 yr 4% MST 6 mo (Resection 28.6 mo, TACE 4 mo, None 5 mo, presence of metastases 4 mo) |
| McAteer et al[15] (2013) SEER database | 238 | Lower hazard of death with surgery (HR = 0.23) and lymphadenectomy (HR = 0.26) More hazard of death with female gender (HR = 2.07), older age (> 5 yr, HR > 5) and distant metastases (HR = 3.4) | Surgery in 112 No surgery in 118 Unknown in 8 | OS 5 yr for 0-4 yr age 53%, 5-19 yr age 32% OS 5 yr for males 40%, females 26% DFS 5 yr for localized 61%, regional 39% and metastatic 9% DFS 5 yr 70% with lymphadenectomy vs 57% without |
| McAteer et al[30] (2013) SEER database | 80 | Lower hazard of death with LT as compared to resection (HR = 0.05) | Surgery (LT 20, resection 60) | OS 5 y with LT 85%, Resection 53% |
| Wang et al[38] (2017) | 65 | Initial treatment allocation predicted OS (TACE HR = 0.298, Resection HR = 0.105 with No treatment as reference) | Resection TACE No treatment | For moderate stage disease: Median OS longer with resection (38 mo) vs TACE (13.6 mo) vs No treatment (1.8 mo). For advanced disease: Median OS longer with TACE (7.1 mo) vs no treatment (2.3 mo) |
| Chemotherapy | ||||
| Czauderna et al[27] (2002) SIOPEL 1 | 39 | Poor outcome related to metastases and higher PRETEXT stage | CT in 37, followed by resection | OS 5 yr 28% EFS 5 yr 17% 93% deaths due to tumour progression |
| Katzenstein et al[16] (2002) CCG/POG | 46 | Poor outcome with recurrent disease Favourable prognosis with stage I and normal AFP Comparable survival between 2 regimens | CT (CDDP + Vincristine + 5-FU vs CDDP + Doxo) | EFS 5 yr 19% (Stage I 88%, III 8%, IV 0) OS 5 yr 19% (Stage I 88%, III 23%, IV 10%) |
| Murawski et al[100] (2016) SIOPEL 2 and 3 | 85 | Complete tumor resection and tumor free margins predict OS | Primary surgery (if feasible) à Super-PLADO (CDDP, Doxo and Carbo) à Assessment for LT | Response to CT in 40% OS at 5 yr 22% 5-yr OS with complete resection 63% vs 59% with LT 5-yr OS with macroscopically involved margins 14% |
| Liver transplantation | ||||
| Reyes et al[101] (2000) | 19 | Risk for recurrence with vascular and LN invasion, distant metastases, size of tumor and male gender | LT ± Systemic or intra-arterial neoadjuvant CT | 1 yr 79% 3 yr 68% 5 yr 63% |
| Austin et al[24] (2006) UNOS database | 41 | Primary cause of death: Metastatic or recurrent disease Pretransplant medical disease and era of LT associated with graft and patient survival | All LT | 1 yr 86% 3 yr 63% 5 yr 58% |
| Arikan et al[13] (2006) | 13 | - | LT in 7 Observation in 6 | Overall 1 yr 53%, 4 yr 27% (With LT 1 yr 72%, 4 yr 72%) No recurrence at 36 mo with LT |
| Beaunoyer et al[23] (2007) | 10 | 1 out of 7 outside MC had recurrence, died | LT in all Pre-LT CT in 5 | OS 1 yR 100%, 5 yR 83% RFS 5 yr 89% |
| Sevmis et al[14] (2008) | 9 | 1 out of 4 outside MC had recurrence, excised | LT in all Pre-LT CT in 3 | 100% survival at 19.8 ± 10.6 (7-32) mo Recurrence in 1 out of 4 outside MC, excised |
| Ismail et al[22] (2009) | 21 | Mortality related to recurrence and PRETEXT stage in the non-LT group, but not in the LT group | LT 11 Non-LT 10 (Resection in 8 - 4 after CT) | OS with LT 72% at median 43 mo and Non-LT 40% at median 66 mo Recurrence after LT in 1/11 and after resection in 6/8 |
| Romano et al[18] (2011) | 10 | - | All primary LT No CT / resection | 80% RFS at median FU of 4 y (1-11 y) |
| Palaniappan et al[26] (2016) | 12 | 1 Multifocal + 2 with microvascular invasion 2 underwent TACE before LT | All primary LT (8 diagnosed incidentally in explant livers) | 92% OS at a median of 5 (1-27) mo |
| Baumann et al[25] (2018) ELTR data | 175 | Survival better in children with inherited liver disease than without (HR = 0.29) and vs adults with HCC with inherited liver disease (HR = 0.27) Survival rate increased with increasing age in non-inherited group | All LT | OS at 5 yr: Patient 58% and Graft 56% Patient survival at 5 yr and 10 yr Inherited: 81% and 81% Non-inherited: 53% and 45% |
AFP: Alpha-fetoprotein; Carbo: Carboplatin; CDDP: Cisplatin; CCG: Children’s cancer group; CT: Chemotherapy; DFS: Disease free survival; EFS: Event-free survival; ELTR: European Liver Transplant Registry; FLHCC: Fibrolamellar variant of HCC; FU: Follow-up; 5-FU: 5-Fluorouracil; HR: Hazard ratio; LN: Lymph-node; LT: Liver transplantation; MC: Milan criteria; MOT: Multi-organ transplantation; MST: Median survival time; OR: Odd’s ratio; OS: Overall survival; PH: Partial hepatectomy; POG: Pediatric Oncology group; PRETEXT: Pretreatment tumor extent evaluation; RFS: Recurrence free survival; SEER: Surveillance epidemiology and end-results; SIOPEL: Group for epithelial liver tumors of the international society of pediatric oncology; TACE: Trans-arterial chemoembolization.
Resection: As per AASLD, resection is only recommended in those adults with a single nodule and CTP class A without evidence of portal hypertension[91]. However, the guidelines differ in the Asia-Pacific region and a more aggressive approach is followed[90]. As per Japanese guidelines, patients with CTP A or B are considered for resection and/or radiofrequency ablation even with 1-3 nodules, up to or more than 3 cm in diameter, but without portal vein invasion or extrahepatic spread[102]. Hong Kong consensus further extends this to suggest resection in intrahepatic portal vein or branch hepatic vein invasion, or with a single extrahepatic metastasis in selected patients, but not with main portal vein invasion[103]. In children, resection rates from the eastern part of the world, where HBV is the commonest cause of pediatric HCC, vary from < 10% to 27%[6,28,29,31,97,98]. With improvement in surgical expertise, the resection rates in pediatric HCC have improved to 40% in the latest series from China with a median survival of more than 30 mo[12,38]. From the SEER database of 60 children with HCC who underwent resection, the 5-year survival rate was 53%[30].
Chemotherapy: There are several trials in adults with HCC where neoadjuvant and adjuvant therapies have been used but none has been convincing enough to translate into a recommendation. The survival rates following chemotherapy in HCC in adults have been dismal. AASLD suggests against the usage of adjuvant therapy following successfully resected or ablated HCC in adults[91]. SIOPEL and POG/CCG have done trials in pediatric HCC on the lines of hepatoblastoma[16,27,100]. SIOPEL-1 study analyzed the outcome of 37 children, who received pre-operative chemotherapy of 1 to 6 PLADO courses (cisplatin and doxorubicin). Response was partial in 49%, while others had either no response or disease progression. Resection was possible in 17 (46%) of these children with successful tumor excision in 36%, while 51% never became resectable. At a median follow-up of 75 mo, 8 (28%) children survived, all with complete resection. There was a dismal outcome of 28% at 5 years. PRETEXT stage and metastases predicted survival in that group of children[27]. In the POG/CCG trial, children with HCC were randomly assigned to receive regimen A (cisplatin, vincristine and fluorouracil) or regimen B (PLADO). There was a poor 5-year event free survival of 19% ± 6% with no difference between the two regimens. Survival and outcome depended on the stage of disease - all 8 stage I patients with complete resection survived post chemotherapy, while 18 (47%) of 38 patients with advanced (stage III or IV) disease had an event (progression, death or new neoplasm) before surgery, and only 2 (10%) of 20 who received chemotherapy became resectable[16]. From the more recent SIOPEL 2 and 3 studies evaluating outcome in 85 children with super PLADO (cisplatin, carboplatin and doxorubicin), 13 patients had upfront surgery. Of the remaining 72, only 29 (40%) showed response to chemotherapy and 39 (46%) never became resectable. Complete resection (including LT) was achievable in 40%. Survival was more with resection or LT (59%) vs not (10%). Tumor-free margin at resection was predictive of good outcome[100]. The results were comparable to the older studies conducted by the German Society for Pediatric Oncology and Hematology (GPOH) where the chemotherapy used were ifosfamide, cisplatin and doxorubicin in HB-89 trial, with addition of carboplatin and ifosfamide in HB-94 trial. The overall survival rates were 33% and 32%, respectively[104]. The more recent trial from GPOH (HB-99) showed 3-year event free and overall survival of children with HCC after primary complete resection followed by 2 cycles of carboplatin/etoposide of 72% and 89%, respectively. However, these figures were 12% and 20% in those with non-resectable malignancy[105]. Cytopenias, vomitings, opportunistic infections, ototoxicity, nephrotoxicity, myocardial toxicity and elevation of transaminases are common side-effects to be looked after[16,27,105].
Sorafenib: This is a novel multikinase inhibitor against Raf kinase and vascular endothelial growth factor receptor, along with anti-proliferative and anti-angiogenic properties. From the multi-center randomized placebo controlled phase III study, usage of Sorafenib in adults with advanced HCC has been shown to improve time to tumor progression (median 5.5 mo vs 2.8 mo) and overall survival (10.7 mo vs 7.9 mo)[106]. Further a randomized controlled study found that the combination of Sorafenib and Doxorubicin in adults was superior to Doxorubicin alone in terms of better overall survival (6.4 mo vs 2.8 mo) and progression-free survival (6.0 mo vs 2.7 mo)[107]. From the German GPOH group, Sorafenib when used in combination with PLADO in children with HCC showed tumor regression in 4 out of 7 unresectable tumors. There was decrease in AFP levels in four children who had very high levels. Hand-foot-skin reaction was seen in 7 (58%) of 12 children[108].
Targeted therapies: Various targeted therapies based on the pathogenic mechanisms of oncogenesis and cell proliferation are under trials in adults with HCC. The common biological pathways for target are VEGF receptor (Sorafenib, Bevacizumab, Brivanib, Sunitinib), epidermal growth factor (Erlotinib), mammalian target of rapamycin (Everolimus), tyrosine kinase receptor for hepatocyte growth factor, cMET (Tivantinib), combined VEGF and cMET (Cabozantinib) and programmed cell death receptor (anti-PD-1, Nivolumab)[2].
Liver transplantation: Liver transplantation offers best cure for HCC in a cirrhotic liver in terms of oncological viewpoint by enabling the widest possible resection margins and simultaneously ensuring complete removal of the diseased liver at risk of developing HCC[90]. The commonest criterion used in adults to decide candidacy for LT is Milan criteria which includes single tumor with diameter less than 5 cm, or tumor foci up to 3 in number each one not exceeding 3 cm, without any vascular invasion or extrahepatic involvement. Application of Milan criteria has been shown to improve the survival after LT in adults from 30% to 75%[91]. However in Asia, where living donors are the mainstay for LT and there are no restrictions imposed by the national organ allocation system, the criteria for LT are more liberal[90]. Most countries like Hong Kong and Taiwan are using extended criteria like University of California San Francisco (UCSF) criteria, i.e., solitary tumor ≤ 65 mm in diameter, or 2-3 tumors, each with diameter ≤ 45 mm and total tumor diameter ≤ 80 mm, and without radiological evidence of vascular invasion or distant metastasis, with acceptable long term survival rates[90,103]. Similarly in Japan, patients beyond Milan criteria or those with recurrent HCC with CTP A or B are sometimes considered for LT[102]. Usage of these criteria is limited to case series in children. Transplantation in children outside Milan criteria has a fairly good outcome of 72%-83% at 5 years[18,22,23]. Various series have shown that the outcome post LT in children with HCC is related to PRETEXT stage, recurrence of disease, vascular and lymph node invasion, size of tumor and distant metastases[10,12,15,16,22,24,27,30,38,99-101]. A recent analysis of ELTR data of 175 pediatric HCC showed that children with inherited liver disease (ILD) have better survival after LT in comparison to children without ILD and adults with ILD[25] (Table 3). There is no head to head prospective series comparing resection vs LT in children with HCC. However as per the SEER database, the 5-year survival rates are better with LT (85%) in contrast to resection (53%) (hazard ratio 0.05, 95%CI: 0.003-0.94)[30].
Radiological interventions: Radiological interventions like radiofrequency ablation (RFA), transarterial embolization (bland), transarterial chemo-embolization (TACE, with doxorubicin drug eluting beads), hepatic arterial infusion chemotherapy (HAIC, with low dose 5-fluorouracil and cisplatin with or without systemic interferon therapy) and transarterial radio-embolization (TARE, using yttrium-90 microspheres) are routinely used in adults with advanced HCC for downstaging and those listed for LT. AASLD suggests using one of the radiological techniques for adult patients (1) listed as per Milan criteria to decrease progression of disease and subsequent dropout from the waiting list, (2) outside Milan to bring them into the LT criteria, and (3) who are not candidates for resection or LT to improve their survival[91]. As HCC nodules receive their blood supply preferentially from the branches of hepatic artery, catheters placed into hepatic artery are used to obliterate tumor vascular supply and also to inject chemotherapeutic agents (TACE). Absolute contraindications for endovascular therapy include surgically resectable tumor, intractable systemic infection, advanced liver disease (CTP > 8 or BCLC stage C), hepatic encephalopathy, lung shunt fraction > 20% and hepatic encephalopathy. Relative contraindications are biliary obstruction, tumor > 10 cm or burden > 50% of liver, bilirubin > 3 mg/dL, albumin < 2 gm/dL, impaired renal function, AST > 5 times upper limit of normal and presence of extrahepatic metastases. Common side effects include elevation of bilirubin and transaminases, and post-embolization syndrome (pain, malaise, low grade fever). In Asian countries, TACE is frequently offered to adults with early HCC, or in combination with ablation when the nodules are more than 3 or exceed 3 cm in diameter[102,103]. There is limited data on radiological interventions in children with HCC[109]. Two series from China comprising 110 children with HCC have shown that overall survival with resection, TACE and no treatment is 29-38, 4-14 and 2-5 mo, respectively[12,38]. Moreover in children with advanced HCC, TACE offered 4.8 mo of survival benefit in comparison to standard treatment[38]. Special considerations related to sedation and procedure should be taken - like there is more risk of post-embolization syndrome and arterial spasm[109].
SURVEILLANCE
AASLD recommends surveillance of adults with cirrhosis using ultrasound with or without AFP every 6 mo in order to improve their survival[91]. Asia-Pacific consensus on HCC suggests an AFP cut-off of > 200 ng/mL for surveillance programs in combination with ultrasound[90]. Compliance to the surveillance guidelines has been shown to improve overall survival in adults (53 mo vs 25 mo) in comparison to non-compliance, and translates into low tumor burden and allocation of curative treatment[110]. For children, we suggest surveillance with ultrasound and AFP every 6 mo for all cirrhotic children and those with chronic HBV infection with elevated transaminases (HBeAg positive or negative), GSD types 1, 3 and 4, alpha-1 antitrypsin deficiency, Wilson disease, autoimmune hepatitis, congenital porto-systemic shunts and hepatic venous outflow tract obstruction. The duration should be reduced to 3 mo in children with BSEP deficiency and tyrosinemia, and increased to 1 year in inactive carriers of HBV[2].
FIBROLAMELLAR HCC
Fibrolamellar variant of HCC (FLHCC) is a distinct rare variant of HCC which is more commonly seen in the pediatric population[8,15,19]. Pathologically, it is characterized by large polygonal cells with abundant eosinophilic cytoplasm containing pale bodies and hyaline globules, and large nuclei surrounded by lamellar stroma and a central scar[19]. There is slight preponderance of females (52%-58%) with a median age ranging between 14 to 33 years. Elevation of AFP is seen in around 10%. A large proportion of these tumors are potentially removable (77%-88%) but recurrence rate as high as 77% has been reported. Up to 60% have extrahepatic metastases. 5-year survival rates after resection and LT range from 58% to 82% and 29% to 55%, respectively and is related to absence of metastases, resectability and old age[111,112]. FLHCC, in contrast to adult HCC, has been shown to have better survival when matched for age, gender, stage of disease and liver functions, but the data is controversial[112]. Pediatric FLHCC cases are older in age (age above 12 yr - 88% vs 29%), less multifocal but more metastatic in contrast to pediatric HCC. Response to chemotherapy (super-PLADO) is partial in 31% and is comparable to Pediatric HCC. Complete resection is possible in 42%, however event free survival (22% vs 28%) and overall survival (42% vs 33%) at 3 years are comparable to pediatric HCC. In pediatric FLHCC younger age (< 12 years) and absence of multifocality are associated with a trend towards better outcome[112].
CONCLUSION
Pediatric HCC is a rare aggressive liver malignancy. Tyrosinemia, perinatal hepatitis-B, familial intrahepatic cholestasis, glycogen storage disorders and congenital portosystemic shunts are common predispositions. Management focuses primarily on early detection and surgery. Recurrence free survival after resection and liver transplantation has improved over last 3 decades. Prevention and surveillance strategies may help in improving the overall outcome. Fibrolamellar variant constitutes one-fourth of pediatric HCC with a variable prognosis.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: India
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: There are no potential conflicts of interest.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: May 14, 2018
First decision: June 15, 2018
Article in press: August 1, 2018
P- Reviewer: Giorgio A, Streba LL, Zhao HT S- Editor: Wang XJ L- Editor: A E- Editor: Yin SY
Contributor Information
Rajeev Khanna, Department of Pediatric Hepatology, Institute of Liver and Biliary Sciences, New Delhi 110070, India. drrajeev_khanna@rediffmail.com.
Sanjeev Kumar Verma, Department of Pediatrics, King George Medical University, Uttar Pradesh 226003, India.
References
- 1.Hadzic N, Finegold MJ. Liver neoplasia in children. Clin Liver Dis. 2011;15:443–462, vii-vix. doi: 10.1016/j.cld.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 2.Kelly D, Sharif K, Brown RM, Morland B. Hepatocellular carcinoma in children. Clin Liver Dis. 2015;19:433–447. doi: 10.1016/j.cld.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 3.Otte JB. Progress in the surgical treatment of malignant liver tumors in children. Cancer Treat Rev. 2010;36:360–371. doi: 10.1016/j.ctrv.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Ng VL, Fecteau A, Shepherd R, Magee J, Bucuvalas J, Alonso E, McDiarmid S, Cohen G, Anand R; Studies of Pediatric Liver Transplantation Research Group. Outcomes of 5-year survivors of pediatric liver transplantation: report on 461 children from a north american multicenter registry. Pediatrics. 2008;122:e1128–e1135. doi: 10.1542/peds.2008-1363. [DOI] [PubMed] [Google Scholar]
- 5.Darbari A, Sabin KM, Shapiro CN, Schwarz KB. Epidemiology of primary hepatic malignancies in US children. Hepatology. 2003;38:560–566. doi: 10.1053/jhep.2003.50375. [DOI] [PubMed] [Google Scholar]
- 6.Lee CL, Ko YC. Survival and distribution pattern of childhood liver cancer in Taiwan. Eur J Cancer. 1998;34:2064–2067. doi: 10.1016/s0959-8049(98)00281-0. [DOI] [PubMed] [Google Scholar]
- 7.Moore SW, Millar AJ, Hadley GP, Ionescu G, Kruger M, Poole J, Stones D, Wainwright L, Chitnis M, Wessels G. Hepatocellular carcinoma and liver tumors in South African children: a case for increased prevalence. Cancer. 2004;101:642–649. doi: 10.1002/cncr.20398. [DOI] [PubMed] [Google Scholar]
- 8.Allan BJ, Wang B, Davis JS, Parikh PP, Perez EA, Neville HL, Sola JE. A review of 218 pediatric cases of hepatocellular carcinoma. J Pediatr Surg. 2014;49:166–171; discussion 171. doi: 10.1016/j.jpedsurg.2013.09.050. [DOI] [PubMed] [Google Scholar]
- 9.Czauderna P. Adult type vs. Childhood hepatocellular carcinoma--are they the same or different lesions? Biology, natural history, prognosis, and treatment. Med Pediatr Oncol. 2002;39:519–523. doi: 10.1002/mpo.10178. [DOI] [PubMed] [Google Scholar]
- 10.Chen JC, Chen CC, Chen WJ, Lai HS, Hung WT, Lee PH. Hepatocellular carcinoma in children: clinical review and comparison with adult cases. J Pediatr Surg. 1998;33:1350–1354. doi: 10.1016/s0022-3468(98)90005-7. [DOI] [PubMed] [Google Scholar]
- 11.Choo SP, Tan WL, Goh BK, Tai WM, Zhu AX. Comparison of hepatocellular carcinoma in Eastern versus Western populations. Cancer. 2016 doi: 10.1002/cncr.30237. [DOI] [PubMed] [Google Scholar]
- 12.Zhang XF, Liu XM, Wei T, Liu C, Li MX, Long ZD, Lv Y. Clinical characteristics and outcome of hepatocellular carcinoma in children and adolescents. Pediatr Surg Int. 2013;29:763–770. doi: 10.1007/s00383-013-3334-4. [DOI] [PubMed] [Google Scholar]
- 13.Arikan C, Kilic M, Nart D, Ozgenc F, Ozkan T, Tokat Y, Yagci RV, Aydogdu S. Hepatocellular carcinoma in children and effect of living-donor liver transplantation on outcome. Pediatr Transplant. 2006;10:42–47. doi: 10.1111/j.1399-3046.2005.00395.x. [DOI] [PubMed] [Google Scholar]
- 14.Sevmis S, Karakayali H, Ozçay F, Canan O, Bilezikci B, Torgay A, Haberal M. Liver transplantation for hepatocellular carcinoma in children. Pediatr Transplant. 2008;12:52–56. doi: 10.1111/j.1399-3046.2007.00777.x. [DOI] [PubMed] [Google Scholar]
- 15.McAteer JP, Goldin AB, Healey PJ, Gow KW. Hepatocellular carcinoma in children: epidemiology and the impact of regional lymphadenectomy on surgical outcomes. J Pediatr Surg. 2013;48:2194–2201. doi: 10.1016/j.jpedsurg.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 16.Katzenstein HM, Krailo MD, Malogolowkin MH, Ortega JA, Liu-Mares W, Douglass EC, Feusner JH, Reynolds M, Quinn JJ, Newman K, et al. Hepatocellular carcinoma in children and adolescents: results from the Pediatric Oncology Group and the Children’s Cancer Group intergroup study. J Clin Oncol. 2002;20:2789–2797. doi: 10.1200/JCO.2002.06.155. [DOI] [PubMed] [Google Scholar]
- 17.Pham TH, Iqbal CW, Grams JM, Zarroug AE, Wall JC, Ishitani MB, Nagorney DM, Moir C. Outcomes of primary liver cancer in children: an appraisal of experience. J Pediatr Surg. 2007;42:834–839. doi: 10.1016/j.jpedsurg.2006.12.065. [DOI] [PubMed] [Google Scholar]
- 18.Romano F, Stroppa P, Bravi M, Casotti V, Lucianetti A, Guizzetti M, Sonzogni A, Colledan M, D’Antiga L. Favorable outcome of primary liver transplantation in children with cirrhosis and hepatocellular carcinoma. Pediatr Transplant. 2011;15:573–579. doi: 10.1111/j.1399-3046.2011.01528.x. [DOI] [PubMed] [Google Scholar]
- 19.Weeda VB, Murawski M, McCabe AJ, Maibach R, Brugières L, Roebuck D, Fabre M, Zimmermann A, Otte JB, Sullivan M, et al. Fibrolamellar variant of hepatocellular carcinoma does not have a better survival than conventional hepatocellular carcinoma--results and treatment recommendations from the Childhood Liver Tumour Strategy Group (SIOPEL) experience. Eur J Cancer. 2013;49:2698–2704. doi: 10.1016/j.ejca.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 20.Nault JC, Zucman-Rossi J. Genetics of hepatocellular carcinoma: the next generation. J Hepatol. 2014;60:224–226. doi: 10.1016/j.jhep.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 21.Kim H, Lee MJ, Kim MR, Chung IP, Kim YM, Lee JY, Jang JJ. Expression of cyclin D1, cyclin E, cdk4 and loss of heterozygosity of 8p, 13q, 17p in hepatocellular carcinoma: comparison study of childhood and adult hepatocellular carcinoma. Liver. 2000;20:173–178. doi: 10.1034/j.1600-0676.2000.020002173.x. [DOI] [PubMed] [Google Scholar]
- 22.Ismail H, Broniszczak D, Kaliciński P, Markiewicz-Kijewska M, Teisseyre J, Stefanowicz M, Szymczak M, Dembowska-Bagińska B, Kluge P, Perek D, et al. Liver transplantation in children with hepatocellular carcinoma. Do Milan criteria apply to pediatric patients? Pediatr Transplant. 2009;13:682–692. doi: 10.1111/j.1399-3046.2009.01062.x. [DOI] [PubMed] [Google Scholar]
- 23.Beaunoyer M, Vanatta JM, Ogihara M, Strichartz D, Dahl G, Berquist WE, Castillo RO, Cox KL, Esquivel CO. Outcomes of transplantation in children with primary hepatic malignancy. Pediatr Transplant. 2007;11:655–660. doi: 10.1111/j.1399-3046.2007.00751.x. [DOI] [PubMed] [Google Scholar]
- 24.Austin MT, Leys CM, Feurer ID, Lovvorn HN 3rd, O’Neill JA Jr, Pinson CW, Pietsch JB. Liver transplantation for childhood hepatic malignancy: a review of the United Network for Organ Sharing (UNOS) database. J Pediatr Surg. 2006;41:182–186. doi: 10.1016/j.jpedsurg.2005.10.091. [DOI] [PubMed] [Google Scholar]
- 25.Baumann U, Adam R, Duvoux C, Mikolajczyk R, Karam V, D’Antiga L, Chardot C, Coker A, Colledan M, Ericzon BG, et al. Survival of children after liver transplantation for hepatocellular carcinoma. Liver Transpl. 2018;24:246–255. doi: 10.1002/lt.24994. [DOI] [PubMed] [Google Scholar]
- 26.Palaniappan K, Borkar VV, Safwan M, Vij M, Govil S, Shanmugam N, Rela M. Pediatric hepatocellular carcinoma in a developing country: Is the etiology changing? Pediatr Transplant. 2016;20:898–903. doi: 10.1111/petr.12754. [DOI] [PubMed] [Google Scholar]
- 27.Czauderna P, Mackinlay G, Perilongo G, Brown J, Shafford E, Aronson D, Pritchard J, Chapchap P, Keeling J, Plaschkes J, et al. Hepatocellular carcinoma in children: results of the first prospective study of the International Society of Pediatric Oncology group. J Clin Oncol. 2002;20:2798–2804. doi: 10.1200/JCO.2002.06.102. [DOI] [PubMed] [Google Scholar]
- 28.Hsu HC, Wu MZ, Chang MH, Su IJ, Chen DS. Childhood hepatocellular carcinoma develops exclusively in hepatitis B surface antigen carriers in three decades in Taiwan. Report of 51 cases strongly associated with rapid development of liver cirrhosis. J Hepatol. 1987;5:260–267. doi: 10.1016/s0168-8278(87)80030-2. [DOI] [PubMed] [Google Scholar]
- 29.Chen WJ, Lee JC, Hung WT. Primary malignant tumor of liver in infants and children in Taiwan. J Pediatr Surg. 1988;23:457–461. doi: 10.1016/s0022-3468(88)80448-2. [DOI] [PubMed] [Google Scholar]
- 30.McAteer JP, Goldin AB, Healey PJ, Gow KW. Surgical treatment of primary liver tumors in children: outcomes analysis of resection and transplantation in the SEER database. Pediatr Transplant. 2013;17:744–750. doi: 10.1111/petr.12144. [DOI] [PubMed] [Google Scholar]
- 31.Lack EE, Neave C, Vawter GF. Hepatocellular carcinoma. Review of 32 cases in childhood and adolescence. Cancer. 1983;52:1510–1515. doi: 10.1002/1097-0142(19831015)52:8<1510::aid-cncr2820520830>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 32.Chang MH, You SL, Chen CJ, Liu CJ, Lee CM, Lin SM, Chu HC, Wu TC, Yang SS, Kuo HS, et al. Decreased incidence of hepatocellular carcinoma in hepatitis B vaccinees: a 20-year follow-up study. J Natl Cancer Inst. 2009;101:1348–1355. doi: 10.1093/jnci/djp288. [DOI] [PubMed] [Google Scholar]
- 33.Chang MH, Chen CJ, Lai MS, Hsu HM, Wu TC, Kong MS, Liang DC, Shau WY, Chen DS. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. Taiwan Childhood Hepatoma Study Group. N Engl J Med. 1997;336:1855–1859. doi: 10.1056/NEJM199706263362602. [DOI] [PubMed] [Google Scholar]
- 34.Chang MH, Shau WY, Chen CJ, Wu TC, Kong MS, Liang DC, Hsu HM, Chen HL, Hsu HY, Chen DS; Taiwan Childhood Hepatoma Study Group. Hepatitis B vaccination and hepatocellular carcinoma rates in boys and girls. JAMA. 2000;284:3040–3042. doi: 10.1001/jama.284.23.3040. [DOI] [PubMed] [Google Scholar]
- 35.Ni YH, Chang MH, Wang KJ, Hsu HY, Chen HL, Kao JH, Yeh SH, Jeng YM, Tsai KS, Chen DS. Clinical relevance of hepatitis B virus genotype in children with chronic infection and hepatocellular carcinoma. Gastroenterology. 2004;127:1733–1738. doi: 10.1053/j.gastro.2004.09.048. [DOI] [PubMed] [Google Scholar]
- 36.Wu JF, Chang MH. Natural history of chronic hepatitis B virus infection from infancy to adult life - the mechanism of inflammation triggering and long-term impacts. J Biomed Sci. 2015;22:92. doi: 10.1186/s12929-015-0199-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takano T, Tajiri H, Hosono S, Inui A, Murakami J, Ushijima K, Miyoshi Y, Etani Y, Abukawa D, Suzuki M, et al. Natural history of chronic hepatitis B virus infection in children in Japan: a comparison of mother-to-child transmission with horizontal transmission. J Gastroenterol. 2017;52:1041–1050. doi: 10.1007/s00535-017-1315-4. [DOI] [PubMed] [Google Scholar]
- 38.Wang J, Mao Y, Liu Y, Chen Z, Chen M, Lao X, Li S. Hepatocellular Carcinoma in Children and Adolescents: Clinical Characteristics and Treatment. J Gastrointest Surg. 2017;21:1128–1135. doi: 10.1007/s11605-017-3420-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Satapathy SK, Garg S, Chauhan R, Malhotra V, Sakhuja P, Sharma BC, Sarin SK. Profile of chronic hepatitis B virus in children in India: experience with 116 children. J Gastroenterol Hepatol. 2006;21:1170–1176. doi: 10.1111/j.1440-1746.2006.04382.x. [DOI] [PubMed] [Google Scholar]
- 40.Iavarone M, Colombo M. HBV infection and hepatocellular carcinoma. Clin Liver Dis. 2013;17:375–397. doi: 10.1016/j.cld.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 41.Liu S, Zhang H, Gu C, Yin J, He Y, Xie J, Cao G. Associations between hepatitis B virus mutations and the risk of hepatocellular carcinoma: a meta-analysis. J Natl Cancer Inst. 2009;101:1066–1082. doi: 10.1093/jnci/djp180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li Z, Xie Z, Ni H, Zhang Q, Lu W, Yin J, Liu W, Ding Y, Zhao Y, Zhu Y, et al. Mother-to-child transmission of hepatitis B virus: evolution of hepatocellular carcinoma-related viral mutations in the post-immunization era. J Clin Virol. 2014;61:47–54. doi: 10.1016/j.jcv.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 43.Mayorandan S, Meyer U, Gokcay G, Segarra NG, de Baulny HO, van Spronsen F, Zeman J, de Laet C, Spiekerkoetter U, Thimm E, et al. Cross-sectional study of 168 patients with hepatorenal tyrosinaemia and implications for clinical practice. Orphanet J Rare Dis. 2014;9:107. doi: 10.1186/s13023-014-0107-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zeybek AC, Kiykim E, Soyucen E, Cansever S, Altay S, Zubarioglu T, Erkan T, Aydin A. Hereditary tyrosinemia type 1 in Turkey: twenty year single-center experience. Pediatr Int. 2015;57:281–289. doi: 10.1111/ped.12503. [DOI] [PubMed] [Google Scholar]
- 45.Larochelle J, Alvarez F, Bussières JF, Chevalier I, Dallaire L, Dubois J, Faucher F, Fenyves D, Goodyer P, Grenier A, et al. Effect of nitisinone (NTBC) treatment on the clinical course of hepatorenal tyrosinemia in Québec. Mol Genet Metab. 2012;107:49–54. doi: 10.1016/j.ymgme.2012.05.022. [DOI] [PubMed] [Google Scholar]
- 46.Luijerink MC, Jacobs SM, van Beurden EA, Koornneef LP, Klomp LW, Berger R, van den Berg IE. Extensive changes in liver gene expression induced by hereditary tyrosinemia type I are not normalized by treatment with 2-(2-nitro-4-trifluoromethylbenzoyl)-1,3-cyclohexanedione (NTBC) J Hepatol. 2003;39:901–909. doi: 10.1016/s0168-8278(03)00433-1. [DOI] [PubMed] [Google Scholar]
- 47.Seda Neto J, Leite KM, Porta A, Fonseca EA, Feier FH, Pugliese R, Miura IK, Chapchap P, Porta G. HCC prevalence and histopathological findings in liver explants of patients with hereditary tyrosinemia type 1. Pediatr Blood Cancer. 2014;61:1584–1589. doi: 10.1002/pbc.25094. [DOI] [PubMed] [Google Scholar]
- 48.Bahador A, Dehghani SM, Geramizadeh B, Nikeghbalian S, Bahador M, Malekhosseini SA, Kazemi K, Salahi H. Liver Transplant for Children With Hepatocellular Carcinoma and Hereditary Tyrosinemia Type 1. Exp Clin Transplant. 2015;13:329–332. doi: 10.6002/ect.2013.0158. [DOI] [PubMed] [Google Scholar]
- 49.Knisely AS, Strautnieks SS, Meier Y, Stieger B, Byrne JA, Portmann BC, Bull LN, Pawlikowska L, Bilezikçi B, Ozçay F, et al. Hepatocellular carcinoma in ten children under five years of age with bile salt export pump deficiency. Hepatology. 2006;44:478–486. doi: 10.1002/hep.21287. [DOI] [PubMed] [Google Scholar]
- 50.Davit-Spraul A, Fabre M, Branchereau S, Baussan C, Gonzales E, Stieger B, Bernard O, Jacquemin E. ATP8B1 and ABCB11 analysis in 62 children with normal gamma-glutamyl transferase progressive familial intrahepatic cholestasis (PFIC): phenotypic differences between PFIC1 and PFIC2 and natural history. Hepatology. 2010;51:1645–1655. doi: 10.1002/hep.23539. [DOI] [PubMed] [Google Scholar]
- 51.Pawlikowska L, Strautnieks S, Jankowska I, Czubkowski P, Emerick K, Antoniou A, Wanty C, Fischler B, Jacquemin E, Wali S, et al. Differences in presentation and progression between severe FIC1 and BSEP deficiencies. J Hepatol. 2010;53:170–178. doi: 10.1016/j.jhep.2010.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Strautnieks SS, Byrne JA, Pawlikowska L, Cebecauerová D, Rayner A, Dutton L, Meier Y, Antoniou A, Stieger B, Arnell H, et al. Severe bile salt export pump deficiency: 82 different ABCB11 mutations in 109 families. Gastroenterology. 2008;134:1203–1214. doi: 10.1053/j.gastro.2008.01.038. [DOI] [PubMed] [Google Scholar]
- 53.Iannelli F, Collino A, Sinha S, Radaelli E, Nicoli P, D’Antiga L, Sonzogni A, Faivre J, Buendia MA, Sturm E, et al. Massive gene amplification drives paediatric hepatocellular carcinoma caused by bile salt export pump deficiency. Nat Commun. 2014;5:3850. doi: 10.1038/ncomms4850. [DOI] [PubMed] [Google Scholar]
- 54.Chen Y, Song X, Valanejad L, Vasilenko A, More V, Qiu X, Chen W, Lai Y, Slitt A, Stoner M, et al. Bile salt export pump is dysregulated with altered farnesoid X receptor isoform expression in patients with hepatocellular carcinoma. Hepatology. 2013;57:1530–1541. doi: 10.1002/hep.26187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou S, Hertel PM, Finegold MJ, Wang L, Kerkar N, Wang J, Wong LJ, Plon SE, Sambrotta M, Foskett P, et al. Hepatocellular carcinoma associated with tight-junction protein 2 deficiency. Hepatology. 2015;62:1914–1916. doi: 10.1002/hep.27872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sambrotta M, Strautnieks S, Papouli E, Rushton P, Clark BE, Parry DA, Logan CV, Newbury LJ, Kamath BM, Ling S, et al. Mutations in TJP2 cause progressive cholestatic liver disease. Nat Genet. 2014;46:326–328. doi: 10.1038/ng.2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vij M, Shanmugam NP, Reddy MS, Govil S, Rela M. Hepatocarcinogenesis in multidrug-resistant P-glycoprotein 3 deficiency. Pediatr Transplant. 2017:21. doi: 10.1111/petr.12889. [DOI] [PubMed] [Google Scholar]
- 58.Reddy SK, Austin SL, Spencer-Manzon M, Koeberl DD, Clary BM, Desai DM, Smith AD, Kishnani PS. Liver transplantation for glycogen storage disease type Ia. J Hepatol. 2009;51:483–490. doi: 10.1016/j.jhep.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 59.Kishnani PS, Austin SL, Abdenur JE, Arn P, Bali DS, Boney A, Chung WK, Dagli AI, Dale D, Koeberl D, et al. Diagnosis and management of glycogen storage disease type I: a practice guideline of the American College of Medical Genetics and Genomics. Genet Med. 2014;16:e1. doi: 10.1038/gim.2014.128. [DOI] [PubMed] [Google Scholar]
- 60.Bianchi L. Glycogen storage disease I and hepatocellular tumours. Eur J Pediatr. 1993;152 Suppl 1:S63–S70. doi: 10.1007/BF02072092. [DOI] [PubMed] [Google Scholar]
- 61.Labrune P, Trioche P, Duvaltier I, Chevalier P, Odièvre M. Hepatocellular adenomas in glycogen storage disease type I and III: a series of 43 patients and review of the literature. J Pediatr Gastroenterol Nutr. 1997;24:276–279. doi: 10.1097/00005176-199703000-00008. [DOI] [PubMed] [Google Scholar]
- 62.Kishnani PS, Chuang TP, Bali D, Koeberl D, Austin S, Weinstein DA, Murphy E, Chen YT, Boyette K, Liu CH, et al. Chromosomal and genetic alterations in human hepatocellular adenomas associated with type Ia glycogen storage disease. Hum Mol Genet. 2009;18:4781–4790. doi: 10.1093/hmg/ddp441. [DOI] [PubMed] [Google Scholar]
- 63.Kishnani PS, Austin SL, Arn P, Bali DS, Boney A, Case LE, Chung WK, Desai DM, El-Gharbawy A, Haller R, et al. Glycogen storage disease type III diagnosis and management guidelines. Genet Med. 2010;12:446–463. doi: 10.1097/GIM.0b013e3181e655b6. [DOI] [PubMed] [Google Scholar]
- 64.Demo E, Frush D, Gottfried M, Koepke J, Boney A, Bali D, Chen YT, Kishnani PS. Glycogen storage disease type III-hepatocellular carcinoma a long-term complication? J Hepatol. 2007;46:492–498. doi: 10.1016/j.jhep.2006.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Townsend SA, Edgar RG, Ellis PR, Kantas D, Newsome PN, Turner AM. Systematic review: the natural history of alpha-1 antitrypsin deficiency, and associated liver disease. Aliment Pharmacol Ther. 2018;47:877–885. doi: 10.1111/apt.14537. [DOI] [PubMed] [Google Scholar]
- 66.Antoury C, Lopez R, Zein N, Stoller JK, Alkhouri N. Alpha-1 antitrypsin deficiency and the risk of hepatocellular carcinoma in end-stage liver disease. World J Hepatol. 2015;7:1427–1432. doi: 10.4254/wjh.v7.i10.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Elzouki AN, Eriksson S. Risk of hepatobiliary disease in adults with severe alpha 1-antitrypsin deficiency (PiZZ): is chronic viral hepatitis B or C an additional risk factor for cirrhosis and hepatocellular carcinoma? Eur J Gastroenterol Hepatol. 1996;8:989–994. doi: 10.1097/00042737-199610000-00010. [DOI] [PubMed] [Google Scholar]
- 68.Marcus NY, Brunt EM, Blomenkamp K, Ali F, Rudnick DA, Ahmad M, Teckman JH. Characteristics of hepatocellular carcinoma in a murine model of alpha-1-antitrypsin deficiency. Hepatol Res. 2010;40:641–653. doi: 10.1111/j.1872-034X.2010.00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Marcus NY, Blomenkamp K, Ahmad M, Teckman JH. Oxidative stress contributes to liver damage in a murine model of alpha-1-antitrypsin deficiency. Exp Biol Med (Maywood) 2012;237:1163–1172. doi: 10.1258/ebm.2012.012106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kamath BM, Baker A, Houwen R, Todorova L, Kerkar N. Systematic Review: the Epidemiology, Natural History and Burden of Alagille Syndrome. J Pediatr Gastroenterol Nutr. 2018;67:148–156. doi: 10.1097/MPG.0000000000001958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bhadri VA, Stormon MO, Arbuckle S, Lam AH, Gaskin KJ, Shun A. Hepatocellular carcinoma in children with Alagille syndrome. J Pediatr Gastroenterol Nutr. 2005;41:676–678. doi: 10.1097/01.mpg.0000179759.60048.c4. [DOI] [PubMed] [Google Scholar]
- 72.Dill MT, Tornillo L, Fritzius T, Terracciano L, Semela D, Bettler B, Heim MH, Tchorz JS. Constitutive Notch2 signaling induces hepatic tumors in mice. Hepatology. 2013;57:1607–1619. doi: 10.1002/hep.26165. [DOI] [PubMed] [Google Scholar]
- 73.Tschaharganeh DF, Chen X, Latzko P, Malz M, Gaida MM, Felix K, Ladu S, Singer S, Pinna F, Gretz N, et al. Yes-associated protein up-regulates Jagged-1 and activates the Notch pathway in human hepatocellular carcinoma. Gastroenterology. 2013;144:1530–1542.e12. doi: 10.1053/j.gastro.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bernard O, Franchi-Abella S, Branchereau S, Pariente D, Gauthier F, Jacquemin E. Congenital portosystemic shunts in children: recognition, evaluation, and management. Semin Liver Dis. 2012;32:273–287. doi: 10.1055/s-0032-1329896. [DOI] [PubMed] [Google Scholar]
- 75.Franchi-Abella S, Branchereau S, Lambert V, Fabre M, Steimberg C, Losay J, Riou JY, Pariente D, Gauthier F, Jacquemin E, et al. Complications of congenital portosystemic shunts in children: therapeutic options and outcomes. J Pediatr Gastroenterol Nutr. 2010;51:322–330. doi: 10.1097/MPG.0b013e3181d9cb92. [DOI] [PubMed] [Google Scholar]
- 76.Sokollik C, Bandsma RH, Gana JC, van den Heuvel M, Ling SC. Congenital portosystemic shunt: characterization of a multisystem disease. J Pediatr Gastroenterol Nutr. 2013;56:675–681. doi: 10.1097/MPG.0b013e31828b3750. [DOI] [PubMed] [Google Scholar]
- 77.Hadžić N, Quaglia A, Portmann B, Paramalingam S, Heaton ND, Rela M, Mieli-Vergani G, Davenport M. Hepatocellular carcinoma in biliary atresia: King’s College Hospital experience. J Pediatr. 2011;159:617–622.e1. doi: 10.1016/j.jpeds.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 78.Tatekawa Y, Asonuma K, Uemoto S, Inomata Y, Tanaka K. Liver transplantation for biliary atresia associated with malignant hepatic tumors. J Pediatr Surg. 2001;36:436–439. doi: 10.1053/jpsu.2001.21600. [DOI] [PubMed] [Google Scholar]
- 79.Moucari R, Rautou PE, Cazals-Hatem D, Geara A, Bureau C, Consigny Y, Francoz C, Denninger MH, Vilgrain V, Belghiti J, et al. Hepatocellular carcinoma in Budd-Chiari syndrome: characteristics and risk factors. Gut. 2008;57:828–835. doi: 10.1136/gut.2007.139477. [DOI] [PubMed] [Google Scholar]
- 80.Khanna R, Alam S, Mukund A, Ahuja A, Rastogi A. Hepatocellular carcinoma in an adolescent with celiac disease. J Pediatr Gastroenterol Nutr. 2013;57:e16–e18. doi: 10.1097/MPG.0b013e3182680d55. [DOI] [PubMed] [Google Scholar]
- 81.Alam S, Lal BB, Sood V, Rawat D. Pediatric Acute-on-Chronic Liver Failure in a Specialized Liver Unit: Prevalence, Profile, Outcome, and Predictive Factors. J Pediatr Gastroenterol Nutr. 2016;63:400–405. doi: 10.1097/MPG.0000000000001179. [DOI] [PubMed] [Google Scholar]
- 82.Tansel A, Katz LH, El-Serag HB, Thrift AP, Parepally M, Shakhatreh MH, Kanwal F. Incidence and Determinants of Hepatocellular Carcinoma in Autoimmune Hepatitis: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2017;15:1207–1217.e4. doi: 10.1016/j.cgh.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hino-Arinaga T, Ide T, Kuromatsu R, Miyajima I, Ogata K, Kuwahara R, Hisamochi A, Torimura T, Sata M; Autoimmune Hepatitis Study Group. Risk factors for hepatocellular carcinoma in Japanese patients with autoimmune hepatitis type 1. J Gastroenterol. 2012;47:569–576. doi: 10.1007/s00535-011-0519-2. [DOI] [PubMed] [Google Scholar]
- 84.van Meer S, de Man RA, van den Berg AP, Houwen RH, Linn FH, van Oijen MG, Siersema PD, van Erpecum KJ. No increased risk of hepatocellular carcinoma in cirrhosis due to Wilson disease during long-term follow-up. J Gastroenterol Hepatol. 2015;30:535–539. doi: 10.1111/jgh.12716. [DOI] [PubMed] [Google Scholar]
- 85.Pfeiffenberger J, Mogler C, Gotthardt DN, Schulze-Bergkamen H, Litwin T, Reuner U, Hefter H, Huster D, Schemmer P, Członkowska A, et al. Hepatobiliary malignancies in Wilson disease. Liver Int. 2015;35:1615–1622. doi: 10.1111/liv.12727. [DOI] [PubMed] [Google Scholar]
- 86.Dragani TA. Risk of HCC: genetic heterogeneity and complex genetics. J Hepatol. 2010;52:252–257. doi: 10.1016/j.jhep.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 87.Zen Y, Vara R, Portmann B, Hadzic N. Childhood hepatocellular carcinoma: a clinicopathological study of 12 cases with special reference to EpCAM. Histopathology. 2014;64:671–682. doi: 10.1111/his.12312. [DOI] [PubMed] [Google Scholar]
- 88.Choi JY, Lee JM, Sirlin CB. CT and MR imaging diagnosis and staging of hepatocellular carcinoma: part II. Extracellular agents, hepatobiliary agents, and ancillary imaging features. Radiology. 2014;273:30–50. doi: 10.1148/radiol.14132362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Roberts LR, Sirlin CB, Zaiem F, Almasri J, Prokop LJ, Heimbach JK, Murad MH, Mohammed K. Imaging for the diagnosis of hepatocellular carcinoma: A systematic review and meta-analysis. Hepatology. 2018;67:401–421. doi: 10.1002/hep.29487. [DOI] [PubMed] [Google Scholar]
- 90.Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, Tateishi R, Han KH, Chawla YK, Shiina S, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11:317–370. doi: 10.1007/s12072-017-9799-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad MH, Marrero JA. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358–380. doi: 10.1002/hep.29086. [DOI] [PubMed] [Google Scholar]
- 92.Jacob J, Deganello A, Sellars ME, Hadzic N, Sidhu PS. Contrast enhanced ultrasound (CEUS) characterization of grey-scale sonographic indeterminate focal liver lesions in pediatric practice. Ultraschall Med. 2013;34:529–540. doi: 10.1055/s-0033-1355785. [DOI] [PubMed] [Google Scholar]
- 93.Darge K, Papadopoulou F, Ntoulia A, Bulas DI, Coley BD, Fordham LA, Paltiel HJ, McCarville B, Volberg FM, Cosgrove DO, et al. Safety of contrast-enhanced ultrasound in children for non-cardiac applications: a review by the Society for Pediatric Radiology (SPR) and the International Contrast Ultrasound Society (ICUS) Pediatr Radiol. 2013;43:1063–1073. doi: 10.1007/s00247-013-2746-6. [DOI] [PubMed] [Google Scholar]
- 94.López-Terrada D, Alaggio R, de Dávila MT, Czauderna P, Hiyama E, Katzenstein H, Leuschner I, Malogolowkin M, Meyers R, Ranganathan S, et al. Towards an international pediatric liver tumor consensus classification: proceedings of the Los Angeles COG liver tumors symposium. Mod Pathol. 2014;27:472–491. doi: 10.1038/modpathol.2013.80. [DOI] [PubMed] [Google Scholar]
- 95.Towbin AJ, Meyers RL, Woodley H, Miyazaki O, Weldon CB, Morland B, Hiyama E, Czauderna P, Roebuck DJ, Tiao GM. 2017 PRETEXT: radiologic staging system for primary hepatic malignancies of childhood revised for the Paediatric Hepatic International Tumour Trial (PHITT) Pediatr Radiol. 2018;48:536–554. doi: 10.1007/s00247-018-4078-z. [DOI] [PubMed] [Google Scholar]
- 96.Wu TC, Tong MJ, Hwang B, Lee SD, Hu MM. Primary hepatocellular carcinoma and hepatitis B infection during childhood. Hepatology. 1987;7:46–48. doi: 10.1002/hep.1840070111. [DOI] [PubMed] [Google Scholar]
- 97.Ni YH, Chang MH, Hsu HY, Hsu HC, Chen CC, Chen WJ, Lee CY. Hepatocellular carcinoma in childhood. Clinical manifestations and prognosis. Cancer. 1991;68:1737–1741. doi: 10.1002/1097-0142(19911015)68:8<1737::aid-cncr2820680815>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 98.Hsiao CC, Chuang JH, Tiao MM, Sheen JM, Shieh CS. Patterns of hepatoblastoma and hepatocellular carcinoma in children after universal hepatitis B vaccination in taiwan: a report from a single institution in southern Taiwan. J Pediatr Hematol Oncol. 2009;31:91–96. doi: 10.1097/MPH.0b013e31818b3784. [DOI] [PubMed] [Google Scholar]
- 99.Tagge EP, Tagge DU, Reyes J, Tzakis A, Iwatsuki S, Starzl TE, Wiener ES. Resection, including transplantation, for hepatoblastoma and hepatocellular carcinoma: impact on survival. J Pediatr Surg. 1992;27:292–296; discussion 297. doi: 10.1016/0022-3468(92)90849-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Murawski M, Weeda VB, Maibach R, Morland B, Roebuck DJ, Zimmerman A, Casanova M, Perilongo G, Laithier V, Kebudi R, et al. Hepatocellular Carcinoma in Children: Does Modified Platinum- and Doxorubicin-Based Chemotherapy Increase Tumor Resectability and Change Outcome? Lessons Learned From the SIOPEL 2 and 3 Studies. J Clin Oncol. 2016;34:1050–1056. doi: 10.1200/JCO.2014.60.2250. [DOI] [PubMed] [Google Scholar]
- 101.Reyes JD, Carr B, Dvorchik I, Kocoshis S, Jaffe R, Gerber D, Mazariegos GV, Bueno J, Selby R. Liver transplantation and chemotherapy for hepatoblastoma and hepatocellular cancer in childhood and adolescence. J Pediatr. 2000;136:795–804. [PubMed] [Google Scholar]
- 102.Kudo M, Matsui O, Izumi N, Iijima H, Kadoya M, Imai Y, Okusaka T, Miyayama S, Tsuchiya K, Ueshima K, et al. JSH Consensus-Based Clinical Practice Guidelines for the Management of Hepatocellular Carcinoma: 2014 Update by the Liver Cancer Study Group of Japan. Liver Cancer. 2014;3:458–468. doi: 10.1159/000343875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Poon RT, Cheung TT, Kwok PC, Lee AS, Li TW, Loke KL, Chan SL, Cheung MT, Lai TW, Cheung CC, et al. Hong Kong consensus recommendations on the management of hepatocellular carcinoma. Liver Cancer. 2015;4:51–69. doi: 10.1159/000367728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.von Schweinitz D, Bürger D, Bode U, Weinel P, Erttmann R, Hecker H, Mildenberger H. [Results of the HB-89 Study in treatment of malignant epithelial liver tumors in childhood and concept of a new HB-94 protocol] Klin Padiatr. 1994;206:282–288. [PubMed] [Google Scholar]
- 105.Schmid I, Albert MH, Ha¨berle B. HB99-Hepatozellula¨re Karzinome: Behandlungsergebnisse und neue Konzepte. Monatsschr Kinderheilkd. 2008;156:412. [Google Scholar]
- 106.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 107.Abou-Alfa GK, Johnson P, Knox JJ, Capanu M, Davidenko I, Lacava J, Leung T, Gansukh B, Saltz LB. Doxorubicin plus sorafenib vs doxorubicin alone in patients with advanced hepatocellular carcinoma: a randomized trial. JAMA. 2010;304:2154–2160. doi: 10.1001/jama.2010.1672. [DOI] [PubMed] [Google Scholar]
- 108.Schmid I, Häberle B, Albert MH, Corbacioglu S, Fröhlich B, Graf N, Kammer B, Kontny U, Leuschner I, Scheel-Walter HG, et al. Sorafenib and cisplatin/doxorubicin (PLADO) in pediatric hepatocellular carcinoma. Pediatr Blood Cancer. 2012;58:539–544. doi: 10.1002/pbc.23295. [DOI] [PubMed] [Google Scholar]
- 109.Lungren MP, Towbin AJ, Roebuck DJ, Monroe EJ, Gill AE, Thakor A, Towbin RB, Cahill AM, Matthew Hawkins C. Role of interventional radiology in managing pediatric liver tumors : Part 1: Endovascular interventions. Pediatr Radiol. 2018;48:555–564. doi: 10.1007/s00247-018-4068-1. [DOI] [PubMed] [Google Scholar]
- 110.Costentin C, Layese R, Bourcier V, Cagnot C, Marcellin P, Guyader D, Pol S, Larrey D, De Lédinghen V, Ouzan D, et al. Compliance With Hepatocellular Carcinoma Surveillance Guidelines Associated With Increased Lead-Time Adjusted Survival of Patients With Compensated Viral Cirrhosis: A Multi-Center Cohort Study. Gastroenterology. 2018;155:431–442.e10. doi: 10.1053/j.gastro.2018.04.027. [DOI] [PubMed] [Google Scholar]
- 111.Ang CS, Kelley RK, Choti MA, Cosgrove DP, Chou JF, Klimstra D, Torbenson MS, Ferrell L, Pawlik TM, Fong Y, et al. Clinicopathologic characteristics and survival outcomes of patients with fibrolamellar carcinoma: data from the fibrolamellar carcinoma consortium. Gastrointest Cancer Res. 2013;6:3–9. [PMC free article] [PubMed] [Google Scholar]
- 112.Mavros MN, Mayo SC, Hyder O, Pawlik TM. A systematic review: treatment and prognosis of patients with fibrolamellar hepatocellular carcinoma. J Am Coll Surg. 2012;215:820–830. doi: 10.1016/j.jamcollsurg.2012.08.001. [DOI] [PubMed] [Google Scholar]