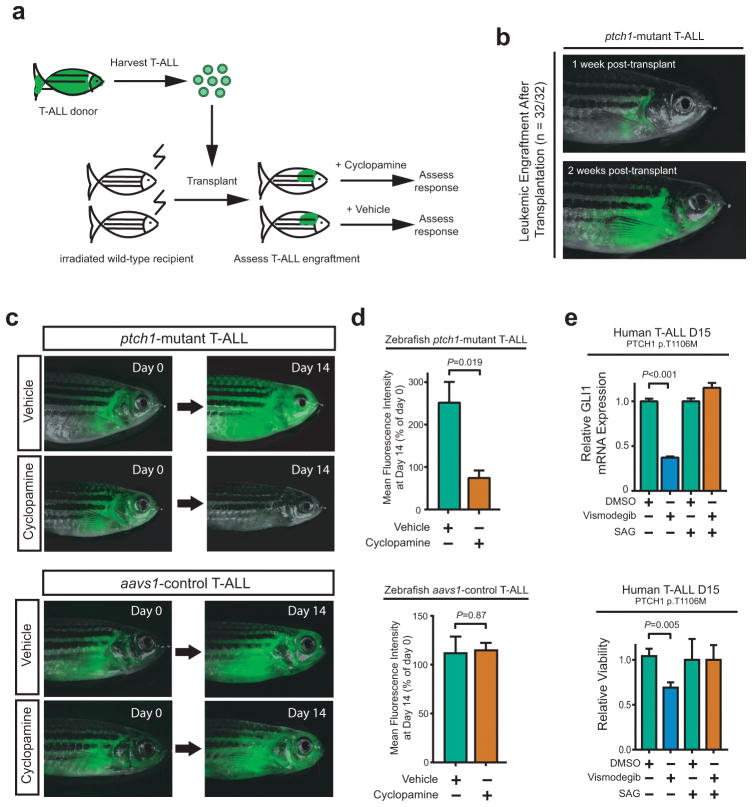
Figure 5. Transplantability of ptch1-mutant T-ALL and therapeutic activity of cyclopamine.
(a) Schema of experimental design. (b) A representative adult ptch1-mutant recipient fish shown at day 7 (top) and 14 (bottom) following transplantation with ptch1; rag2-notch1aICD T-ALL. All 32 transplanted ptch1-mutant zebrafish demonstrated similar leukemic engraftment and rapid disease progression. Following leukemic engraftment, fish were treated with 1μM cyclopamine or vehicle control (ethanol) for 14 days. (c) Representative zebrafish in cyclopamine-treated and vehicle control (ethanol) for ptch1-mutant (top panel) and aavs1-control zebrafish (bottom panel) at the indicated time points. (d) Leukemic burden was assessed by mean fluorescence intensity (MFI), with MFI at day 14 normalized to day 0 for each individual fish. Of note, 5 ptch1-mutant fish from each group (vehicle and cyclopamine) met criteria for euthanasia due to radiation toxicity by day 14, and most others became ill soon thereafter, thus the study was terminated at this time point. A two-sided Welch t-test was used to assess differences among treatment groups. (Number of fish per group: ptch1-mutant recipient fish - Day 0, vehicle=10, cyclopamine=11; Day 14, vehicle=5, cyclopamine=5; aavs1-control recipient fish - Day 0, vehicle=9, cyclopamine=11; Day 14, vehicle=8, cyclopamine=9). (e) Cells from a primary T-ALL patient sample that harbored a p.T1106M PTCH1 mutation, which had been engrafted and expanded in immunodeficient mice, were treated with DMSO control, 10nM vismodegib, or 100nM SAG. After 48 hours, (top panel) relative GLI1 mRNA expression (to β-Actin control) and (bottom panel) viability using trypan blue staining were assessed. Results were normalized to DMSO control. Differences were assessed using a Welch t-test.