Table 1. Optimization of the reaction conditions a .
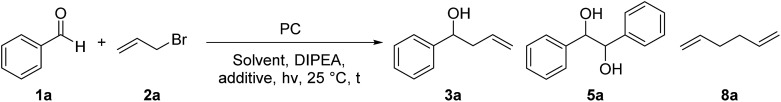
| ||||||||
| Entry | PC (mol%, hν, [nm]) | Solvent | DIPEA (eq.) | Additive (eq.) | t [h] | Yield 3a b [%] | Yield 5a c [%] | Yield 8a d [%] |
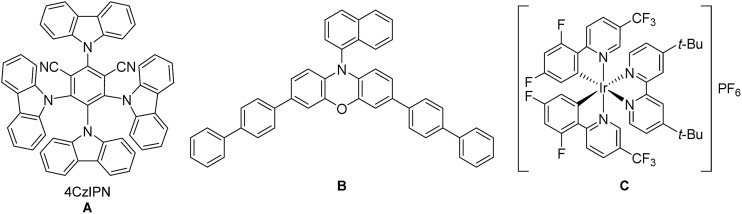
| 1 | A (5, 455) | DMF (dry) | 6 | — | 18 | 22 | 15 | 31 |
| 2 | B (5, 455) | DMF (dry) | 6 | — | 18 | 38 | 8 | 17 |
| 3 | B (5, 400) | DMA | 6 | — | 18 | 54 | 14 | 23 |
| 4 | C (2, 455) | DMA | 6 | — | 18 | 21 | 19 | 43 |
| 5 | B (5, 400) | DMA | 6 | LiBF4 (1.5) | 18 | 64 | 13 | 23 |
| 6 | B (5, 400) | DMA | 6 | LiBF4 (1.5) | 2 | 59 | 12 | 28 |
| 7 | B (5, 400) | DMA | — | LiBF4 (1.5) | 18 | 0 | 0 | 0 |
| 8 | B (5, dark) | DMA | 6 | LiBF4 (1.5) | 18 | 0 | 0 | 0 |
| 9 | — (400) | DMA | 6 | LiBF4 (1.5) | 15 | 46 | 3 | 15 |
| 10 | — (400) | DMA | 6 | LiBF4 (1.5) | 4 | 3 | 2 | 6 |
| 11 | — (455) | DMA | 6 | LiBF4 (1.5) | 15 | 0 | 0 | 0 |
| ||||||||
aThe reactions were performed using 1 eq. (0.2 mmol) 1a and 2 eq. (0.4 mmol) 2a in 2 mL degassed solvent under nitrogen.
bYields were determined by GC analysis with 1-naphthol as an internal standard.
cYields were determined by crude NMR with 1,3,5-trimethoxybenzene as an internal standard.
dYields were determined by GC analysis with 1-naphthol as an internal standard.
