Table 2.
Representative examples of the α-oxidation of cyclic ketones.
| Entry | Substrate | Conditions | Product (Yield) | Reference |
| 1 | 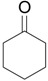 |
1.1 eq. o-H2OCC6H4IO, 3 eq. KOH, MeOH, rt, overnight | 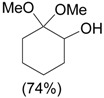 |
Moriarty et al. [28] |
| 2 | 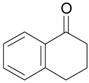 |
1.1 eq. PhI(OAc)2, 3 eq. KOH, MeOH, 0-5 °C for 1 h and 23-25 °C for 20 h | 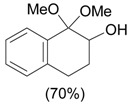 |
Moriarty et al. [29] |
| 3 | 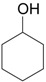 |
3.0 eq. PhIO, 2.5 eq. p-TsOH.H2O, CH3CN, 60 °C, 3 h | 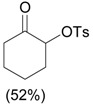 |
Ueno et al. [30] |
| 4 |  |
PhI(OAc)2, p-TsOH grinding, 20 min |
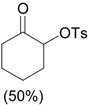 |
Yusubov and Wirth [31] |
| 5 |  |
i) 0.33 eq. PhIO, L-proline (10-30 mol %), DMF, rt, 16-24 h; ii) NaBH4, MeOH, 0 °C |
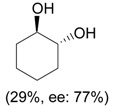 |
Engqvist et al. [32] |
