Abstract
Tetralogy of Fallot (TOF) is a congenital heart disease characterized by abnormal cardiomyocyte differentiation in the right ventricular outflow tract (RVOT), and HA117 is a novel long noncoding RNA (lncRNA) with anti-differentiation roles.
To investigate the potential association of HA117 with TOF, we collected 84 RVOT tissues from patients with TOF. We determined the expression of HA117 in RVOT samples from TOF patients and collected clinical data to conduct a cross-sectional and short-term follow-up study.
McGoon ratio, Nakata index, and left ventricular end-diastolic volume index (LVEDVI) were negatively correlated with the expression of HA117 based on subgroup analysis, correlation analysis and logistic regression analysis. Additionally, cardiopulmonary bypass (CPB) time and ICU stay were longer in patients with higher expression of HA117 than in patients with lower expression of HA117. Furthermore, percentage improvement in SPO2 was significantly reduced in patients with increased HA117 expression at 6 months after surgery.
Our results suggested that the increased expression of the novel lncRNA HA117 is a risk factor for unfavorable McGoon ratio, Nakata index and LVEDVI in TOF patients. Additionally, an increased expression of HA117 might lead to adverse short-term outcomes in TOF patients.
Keywords: HA117, Tetralogy of Fallot, Long noncoding RNA, McGoon ratio, Nakata index
Introduction
Congenital heart disease (CHD) is one of the most common congenital malformations with a morbidity of 8‰, and 28% of congenital malformations are accompanied by cardiac abnormalities.1, 2 Tetralogy of Fallot (TOF) is a typical complex CHD and accounts for 3%–5% of all CHD cases.3 Deformities of the right ventricular outflow tract (RVOT), especially in pulmonary artery, are observed in almost all TOF patients,4 and the indexes of severity of RVOT dysplasia such as McGoon ratio, Nakata index and left ventricular end-diastolic volume index (LVEDVI) are predictors of prognosis.5 A relationship between abnormal differentiation of cardiomyocytes and deformities of the RVOT has been widely reported by several recently studies,6, 7 with a focus on several genes and noncoding RNAs associated with cell differentiation.8, 9
HA117 (also known as NS117) is a novel long noncoding RNA (lncRNA) (GenBank accession number: CB214920) located in the 14q24.2 region of chromosome 14. We first reported HA117 in multidrug-resistant subpopulations of HL60 cells (a human acute promyelocytic leukemia cell line) induced by all-trans retinoic acid (ATRA).10 Our previous studies indicated that HA117 is closely related to cell differentiation in vivo and in vitro.11, 12, 13
Here, we determined the expression of HA117 in RVOT samples from TOF patients and collected clinical data to conduct a cross-sectional and short-term follow-up study. Our study aimed to investigate the correlation between HA117 expression and TOF, including both severity and prognosis of TOF.
Methods
Study participants and data collection
We enrolled patients undergoing total correction of TOF at the Department of Cardiothoracic surgery, Children's Hospital of Chongqing Medical University from January 2016 to March 2017 and determined HA117 expression by real-time polymerase chain reaction (QPCR) at Chongqing Key Laboratory of Pediatrics, Chongqing Medical University. Forward primer of HA117 for QPCR was 5′-CAGAGTCAGGGACTTCAGCCTTAT-3′ and reverse primer was 5′-CTGTTTCCTTCTCACTCCCAACCA-3′. In situ hybridization (ISH) was used to ascertain the expression of HA117 in RVOT tissues by random inspection. Forward primer of HA117 for ISH was 5′-TCTCTACCTACCAGGATGAGTCCAATGCC-3′ and reverse primer was 5′-TCAGGGACTTCAGCCTTATGGTGACA-3′.
Patients with adequate information on baseline characteristics in our Cardiovascular Treatment System (CTS) database were included in the current study. Patients with previous heart surgery or combined chest wall deformity or patients who were lost to follow-up were excluded. A total of 84 individuals (55 males and 29 females) were included in this study.
Data on baseline demographic and clinical characteristics, including age, sex, HA117 expression, McGoon ratio, Nakata index, LVEDVI, left ventricular ejection fraction (LVEF), SPO2, pulmonary artery valve reflux grade (PR grade), tricuspid valve reflux grade (TR grade), cardiopulmonary bypass (CPB) time, aortic cross-clamp (ACC) time, respirator time, ICU stay, death, and organ system dysfunction, were collected from the CTS database. Improvement in LVEF or SPO2, PR grade, TR grade and ventricular arrhythmia at 6 months post-surgery were also examined. McGoon ratio was calculated as (diameter of the left pulmonary artery + diameter of the right pulmonary artery)/(diameter of the aorta on diaphragmatic level; Nakata index was calculated as area of the left pulmonary artery + area of the right pulmonary artery)/(body surface area); LVEDVI was calculated as (left ventricular end-diastolic volume)/(body surface area)14; and body surface area (m2) was calculated as (0.0061 × height (cm) + 0.0124 × weight (kg)–0.0099).15 Other data were obtained directly from routine examination such as echocardiography, cardiac CT or electrocardiography.
All RVOT samples were resected from TOF patients during surgery at the Department of Cardiothoracic Surgery, Children's Hospital, Chongqing Medical University. This study was approved by the Ethical Committee of Chongqing Medical University to ensure the protection of the patients' privacy. Families of the patients agreed to the study and signed consent forms.
Real-time polymerase chain reaction (QPCR)
Total RNA was extracted from RVOT tissues or cell lines with TRIzol reagent (Sigma, St. Louis, MO, USA). Then, first-strand cDNA was synthesized from RNA by reverse transcription using a PrimeScript™ RT reagent kit (TaKaRa, JPN). Real-time PCR was performed using the CXF96 (Bio-Rad, USA) system and SYBR Green Real-Time PCR kit (TaKaRa, JPN). The PCR conditions were as follows: denaturation at 95 °C for 3 min, followed by 35 cycles at 95 °C (30 s), 59 °C (30 s) and 72 °C (30 s), and a final extension at 72 °C for 5 min.
Diagnosis criteria and data grade
Organ system dysfunction was diagnosed according to previously reported criterion (Supplementary Table. s1).16 PR grade and TR grade were defined as no reflux (grade 0), severe reflux (grade 1), moderate reflux (grade 2), severe reflux (grade 3) and extremely severe reflux (grade 4) according to echocardiography results. McGoon ratio, Nakata index, LVEDVI, LVEF and SPO2 were divided into two groups each using cut-off values 1.2, 150 mm2/m2, 30 mL/m2, 50 and 80, respectively according to previously published studies.5
Statistical analysis
Continuous data were presented as the mean with standard deviation (SD) or median with interquartile range (IQR). Categorical variables were presented as proportions. Continuous data were compared using unpaired t-tests or Mann–Whitney U tests. Chi-square test was used to compare categorical variables between two groups. Pearson's or Spearman's correlation analysis was used to determine the correlation between HA117 expression and other clinical indicators. Logistic regression analysis was used to examine whether HA117 was an independent predictor of McGoon ratio, Nakata index, LVEDVI, LVEF, SPO2, PR grade and TR grade. All analyses were performed with SPSS, version 24.0 and GraphPad Prism version 5.0. P value of less than 0.05 was considered statistically significant.
Results
Baseline clinical characteristics and preoperative data
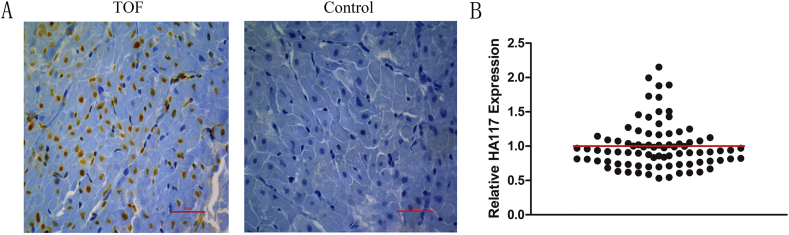
The expression of HA117 in RVOT tissues was detected by QPCR and confirmed by ISH (Fig. 1). Patients were divided into two groups according to the relative expression of HA117, with 1.0 set as the cut-off. In the whole cohort, 32 of 84 (55 males and 29 females) patients had relative HA117 expression of more than 1.0. No significant difference in age or sex was found between patients with higher HA117 expression and patients with lower HA117 expression. Additionally, McGoon ratio, Nakata index, LVEDVI and LVEF in patients in the higher HA117 expression group were significantly lower than those in patients in the lower HA117 expression group (p < 0.05, Table 1).
Figure 1.
Expression of HA117 in RVOT tissues from TOF patients. (A) Expression of HA117 detected by in situ hybridization (ISH). (B) Relative expression of HA117 determined by real-time polymerase chain reaction (QPCR).
Table 1.
Demographic and preoperative data.
| Variable | Total (n = 84) | HA117 ≥ 1.0 (n = 32) | HA117 < 1.0 (n = 52) | p-Value |
|---|---|---|---|---|
| Age (months)a | 13 (9,24.5) | 14 (10.25,25.75) | 12 (9.00,20.75) | 0.381 |
| Male (n, %) | 55 (65.48%) | 19 (59.38%) | 36 (69.23%) | 0.483 |
| Mcgoon ratio | 1.47 ± 0.30 | 1.32 ± 0.27 | 1.56 ± 0.28 | <0.001∗ |
| Nakata index (mm2/m2) | 146.50 ± 30.28 | 133.10 ± 26.72 | 154.80 ± 29.58 | 0.001∗ |
| LVEDVI(mL/m2) | 35.48 ± 7.53 | 37.48 ± 7.15 | 32.24 ± 6.09 | 0.002∗ |
| LVEF (%) | 60.29 ± 5.63 | 61.34 ± 5.46 | 58.59 ± 5.57 | 0.029∗ |
| SPO2 (%) | 76.79 ± 7.37 | 77.37 ± 7.58 | 75.86 ± 7.04 | 0.360 |
| PR grade ≥ III (n, %) | 30 (35.71%) | 15 (46.88%) | 15 (28.85%) | 0.113 |
| TR grade ≥ III (n, %) | 14 (16.67%) | 8 (25.00%) | 6 (11.54%) | 0.761 |
LVEDVI: Left Ventricular End-Diastolic Volume Index; LVEF: Left Ventricular Ejection Fraction.
PR grade: Pulmonary Artery Valve Reflux Grade; TR grade: Tricuspid Valve Reflux Grade.
∗: p < 0.05.
Variable was not coincided with normal distribution and was shown as form of IQR (Interquartile Range).
Moreover, McGoon ratio (r = −0.380, p = 0.010), Nakata index (r = −0.256, p = 0.019), LVEDVI (r = −0.120, p = 0.044) and LVEF (r = −0.135, p = 0.032) were negatively correlated with HA117 expression (Table 2).
Table 2.
Correlation of variable and HA117 expression.
| Variable | r | p-Value |
|---|---|---|
| Mcgoon ratio | −0.380 | 0.010∗ |
| Nakata index (mm2/m2) | −0.256 | 0.019∗ |
| LVEDVI(mL/m2) | −0.120 | 0.044∗ |
| LVEF (%) | −0.135 | 0.032∗ |
| SPO2 (%) | −0.211 | 0.055 |
LVEDVI: Left Ventricular End-Diastolic Volume Index; LVEF: Left Ventricular Ejection Fraction.
∗: p < 0.05.
Univariate logistic regression analysis showed that McGoon ratio (odds ratio (OR) = 5.246, 95% confidence interval (CI): 1.737–15.841, p = 0.003), Nakata index (OR = 3.857, 95% CI: 1.420–10.476, p = 0.008) and LVEDVI (OR = 4.853, 95% CI: 1.742–13.519, p = 0.003) were associated with HA117 expression. Multivariate logistic regression analysis further confirmed the association between Nakata index and HA117 expression (OR = 5.405, 95% CI: 1.765–16.559, p = 0.003) after adjustment for sex (Table 3).
Table 3.
Logistic regression analysis of HA117 and variable.
| Variable | Subgroup | Univariate logistic regression |
Multivariate logistic regression |
||
|---|---|---|---|---|---|
| OR (95%CI) | p-Value | OR (95%CI) | p-Value | ||
| Mcgoon ratio (≥1.2) | age | 1.022 (0.986,1.059) | 0.227 | ||
| gender | 2.344 (0.698,7.866) | 0.168 | |||
| HA117 (g) | 5.246 (1.737,15.841) | 0.003∗ | |||
| HA117 | 7.025 (1.569,31.443) | 0.011∗ | |||
| Nakata index (≥150 mm2/m2) | age | 0.303 (0.983,1.057) | 0.303 | ||
| gender | 3.167 (1.244,8.062) | 0.016∗ | 4.553 (1.563,13.264) | 0.005∗ | |
| HA117 (g) | 3.857 (1.420,10.476) | 0.008∗ | 5.405 (1.764,16.559) | 0.003∗ | |
| HA117 | 3.438 (0.771,15.327) | 0.105∗ | |||
| LVEDVI (≥30 mL/m2) | age | 1.017 (0.983,1.053) | 0.323 | ||
| gender | 1.715 (0.591,4.975) | 0.321 | |||
| HA117 (g) | 4.853 (1.742,13.519) | 0.003∗ | |||
| HA117 | 4.957 (1.193,20.595) | 0.028∗ | |||
| LVEF (≥50%) | age | 1.071 (1.009,1.137) | 0.024∗ | ||
| gender | 1.057 (0.092,12.168) | 0.965 | |||
| HA117 (g) | 3.401 (0.296,39.104) | 0.326 | |||
| HA117 | 7.313 (0.553,96.637) | 0.131 | |||
| SPO2 (≥80%) | age | 0.974 (0.941,1.008) | 0.128 | ||
| gender | 0.391 (0.137,1.116) | 0.079 | |||
| HA117 (g) | 1.471 (0.566,3.824) | 0.428 | |||
| HA117 | 3.637 (0.722,18.330) | 0.118 | |||
| PR grade (≥III) | age | 0.987 (0.952,1.023) | 0.476 | ||
| gender | 1.086 (0.423,2.786) | 0.864 | |||
| HA117 (g) | 2.176 (0.869,5.448) | 0.097 | |||
| HA117 | 3.981 (1.002,15.820) | 0.497 | |||
| TR grade (≥III) | age | 0.991 (1.002,15.820) | 0.707 | ||
| gender | 0.652 (0.202,2.103) | 0.474 | |||
| HA117 (g) | 2.556 (0.795,8.217) | 0.115 | |||
| HA117 | 4.192 (0.904,19.447) | 0.067 | |||
∗: p < 0.05.
HA117 (g): Expressions of HA117 were divided into two groups by cut-off of 1.0, the data was recognized as a categorical variable.
LVEDVI: Left Ventricular End-Diastolic Volume Index; LVEF: Left Ventricular Ejection Fraction.
PR grade: Pulmonary Artery Valve Reflux Grade; TR grade: Tricuspid Valve Reflux Grade.
If age or gender were statistical difference in single factor analysis, the multiple factor analysis was calculated to eliminate the interference of covariate.
Perioperative data
Patients with higher HA117 expression had longer CPB time (127.50 ± 30.56 vs. 109.9 ± 40.92, p = 0.036) and ICU stay (107.10 ± 26.66 vs. 94.41 ± 24.03, p = 0.041) than patients with lower HA117 expression. The mortality within the perioperative period was 9.52% (7/84), 12.50% (4/32), and 5.77% (3/52) in all patients, patients with higher HA117 expression and patients with lower HA117 expression, respectively (p = 0.423). There was no significant difference in ACC time, respirator time or prevalence of organ dysfunction (Table 4).
Table 4.
Perioperative data.
| Variable | Total (n = 84) | HA117 ≥ 1.0 (n = 32) | HA117 < 1.0 (n = 52) | p-Value |
|---|---|---|---|---|
| CPB(min) | 116.80 ± 38.13 | 127.50 ± 30.56 | 109.9 ± 40.92 | 0.036∗ |
| ACC(min) | 88.71 ± 25.60 | 92.78 ± 24.60 | 86.20 ± 25.02 | 0.254 |
| Respirator time(h) | 30.17 (25.0,34.99) | 30.82 (25.00,35.27) | 29.76 (24.73,33.40) | 0.617 |
| ICU time(h) | 102.50 ± 26.30 | 107.10 ± 26.66 | 94.41 ± 24.03 | 0.041∗ |
| Death (n, %) | 7 (9.52%) | 4 (12.50%) | 3 (5.77%) | 0.423 |
| Organ dysfunction (n, %) | 24 (28.57%) | 9 (28.13%) | 15 (28.85%) | 0.950 |
∗: p < 0.05.
CPB: Cardiopulmonary Bypass; ACC (aortic cross-clamp).
Postoperative data at 6 months after surgery
Postoperative data were collected at 6 months after surgery. Compared to the preoperative baseline value, the percentage improvement in SPO2 at 6 months after surgery was 15.4% (2.80, 17.5), 2.4% (2.1, 16.7), and 16.3% (3.3, 25.4) in all patients, patients with higher HA117 expression and patients with lower HA117 expression, respectively, and the difference was significant (p = 0.029). The prevalence of PR grade ≥ II, TR grade ≥ II and ventricular arrhythmia was slightly higher in patients with higher HA117 expression than in patients with lower HA117 expression; however, the difference did not reach significance (Table 5).
Table 5.
Postoperative data of 6 months after surgery.
| Variable | Total (n = 77) | HA117 ≥ 1.0 (n = 28) | HA117 < 1.0 (n = 49) | p-Value |
|---|---|---|---|---|
| SPO2 change (%)a | 15.4 (2.80, 17.5) | 2.4 (2.1, 16.7) | 16.3 (3.3, 25.4) | 0.029∗ |
| PR grade ≥ II (n, %) | 15 (19.48%) | 8 (28.57%) | 7 (14.29%) | 0.143 |
| TR grade ≥ II (n, %) | 8 (10.39%) | 5 (17.86%) | 3 (6.12%) | 0.132 |
| Ventricular arrhythmia (n, %) | 5 (6.49%) | 3 (10.71%) | 2 (4.09%) | 0.357 |
∗: p < 0.05.
SPO2 change (%): Percent of SPO2 improvement compared to preoperative data; PR grade: Pulmonary Artery Valve Reflux Grade; TR grade: Tricuspid Valve Reflux Grade.
Variable was not coincided with normal distribution and was shown as form of IQR (Interquartile Range).
Discussion
Because of anatomic abnormality in TOF, especially the overriding of aorta and obstruction of RVOT, dysplasia of the left ventricle and pulmonary artery is common. McGoon ratio, Nakata index and LVEDVI are the main standardized indicators of the development of left ventricle and pulmonary artery dysplasia. Reduced values of the McGoon ratio, Nakata index and LVEDVI are predictors of adverse outcomes.5, 17, 18
TOF is a severe CHD that leads to numerous complications and poor outcomes in several patients.19, 20 Therefore, research on the etiology of TOF is necessary and urgent. Abnormalities in cardiomyocyte differentiation in RVOT tissues from TOF patients can be observed throughout the entire period of heart development,21 and several genes related to cell differentiation play roles in TOF.9, 22
HA117 was first discovered in an experiment in which HL60 cells were induced with ATRA; HA117 is considered to be a gene segment related to cell differentiation.10 HA117 was later found to display anti-differentiation functions in leukemia cells, solid tumors and Hirschsprung's disease.11, 12, 13 DPF3 and RGS6 are genes neighboring HA117, and these two genes are regulated by HA117 and play important roles in the heart tissues. DPF3 plays a complex role in TOF; it is expressed in the heart during early embryonic stages, and its level gradually decreases after heart formation.23 RGS6 is a dominant regulator of G protein signaling modulators involved in parasympathetic regulation in the heart.24 Considering the function of HA117 reported before and during abnormal cardiomyocyte differentiation in TOF patients, we hypothesized that the severity of TOF might be related to the expression of HA117. To confirm this hypothesis, we performed this cross-sectional and short-term follow-up study.
Our study included 84 patients who underwent total correction of TOF. Our data displayed that the higher was the expression of HA117, the lower were the values of clinical indices and worse was the heart function. To further investigate the association between clinical indexes and HA117 expression, we performed correlation analysis in all patients. The results showed that HA117 expression was negatively correlated with McGoon ratio, Nakata index, LVEDVI and LVEF. We further performed logistic regression analysis and found that an increased HA117 expression was a risk factor for unfavorable McGoon ratio, Nakata index and LVEDVI.
We also observed that the CPB time and ICU stay were longer for patients with higher expression of HA117 than in patients with lower expression of HA117. The percentage improvement in SPO2 was also significantly reduced in patients with higher HA117 expression at 6 months after surgery. Other data obtained during the perioperative period and 6 months after surgery also showed that increased HA117 expression was a risk factor. Although the difference was not significant, the results show that higher HA117 expression can lead to adverse outcomes in TOF patients to some extent.
Nevertheless, some limitations of this study should be mentioned. First, we cannot establish a causal relationship between HA117 expression and long-term prognosis, as we followed up the patients for merely 6 months after surgery. Second, the mechanism of action of HA117 in TOF is not clear. Further in vitro studies are thus necessary. However, to the best of our knowledge, this is the first study on the expression of a novel lncRNA, HA117, in TOF.
Conclusions
Increased HA117 expression is a risk factor for unfavorable McGoon ratio, Nakata index and LVEDVI in TOF patients. Additionally, increased expression of HA117 might indicate adverse short-term outcomes in TOF patients.
Conflicts of interest
The authors declare that no conflicts of interest exist.
Acknowledgements
The study was funded by the National Natural Science Foundation of China (http://www.nsfc.gov.cn/) (NSFC 81370474 and NSFC 81400577). The funding agency had no role in study design, reagent and material acquisition, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Peer review under responsibility of Chongqing Medical University.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.gendis.2018.03.002.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Bernier P.L., Stefanescu A., Samoukovic G. The challenge of congenital heart disease worldwide: epidemiologic and demographic facts. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2010;13(1):26–34. doi: 10.1053/j.pcsu.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 2.Dolk H., Loane M., Garne E. European surveillance of congenital anomalies working G. congenital heart defects in Europe: prevalence and perinatal mortality, 2000 to 2005. Circulation. 2011;123(8):841–849. doi: 10.1161/CIRCULATIONAHA.110.958405. [DOI] [PubMed] [Google Scholar]
- 3.Van der Linde D., Konings E.E., Slager M.A. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol. 2011;58(21):2241–2247. doi: 10.1016/j.jacc.2011.08.025. [DOI] [PubMed] [Google Scholar]
- 4.Apitz C., Webb G.D., Redington A.N. Tetralogy of Fallot. Lancet. 2009;374(9699):1462–1471. doi: 10.1016/S0140-6736(09)60657-7. [DOI] [PubMed] [Google Scholar]
- 5.Zhao K., Wang H., Han H. Staged procedures versus primary repair for tetralogy of Fallot and small left ventricle. Heart Surg Forum. 2012;15(1):E37–E39. doi: 10.1532/HSF98.20111103. [DOI] [PubMed] [Google Scholar]
- 6.Musunuru K., Domian I.J., Chien K.R. Stem cell models of cardiac development and disease. Annu Rev Cell Dev Biol. 2010;26:667–687. doi: 10.1146/annurev-cellbio-100109-103948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paige S.L., Plonowska K., Xu A. Molecular regulation of cardiomyocyte differentiation. Circ Res. 2015;116(2):341–353. doi: 10.1161/CIRCRESAHA.116.302752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldmuntz E., Woyciechowski S., Renstrom D. Variants of folate metabolism genes and the risk of conotruncal cardiac defects. Circ Cardiovasc Genet. 2008;1(2):126–132. doi: 10.1161/CIRCGENETICS.108.796342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xia Y., Hong H., Ye L. Label-free quantitative proteomic analysis of right ventricular remodeling in infant tetralogy of Fallot patients. J Proteomics. 2013;84:78–91. doi: 10.1016/j.jprot.2013.03.032. [DOI] [PubMed] [Google Scholar]
- 10.Zheng G.H., Fu J.R., Xu Y.H. Screening and cloning of multi-drug resistant genes in HL-60/MDR cells. Leuk Res. 2009;33(8):1120–1123. doi: 10.1016/j.leukres.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 11.Duan W., Jin X., Xiu Y. Expression of the novel all-trans retinoic acid-related resistance gene HA117 in pediatric solid tumors. J Pediatr Hematol Oncol. 2014;36(1):45–50. doi: 10.1097/MPH.0b013e31828e5d73. [DOI] [PubMed] [Google Scholar]
- 12.Li S., Jin X., Wu H. HA117 endows HL60 cells with a stem-like signature by inhibiting the degradation of DNMT1 via its ability to down-regulate expression of the GGL domain of RGS6. PLoS One. 2017;12(6):e0180142. doi: 10.1371/journal.pone.0180142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu H., Luo Y., Li S. Expression profiles of HA117 and its neighboring gene DPF3 in different colon segments of Hirschsprung's disease. Int J Clin Exp Pathol. 2014;7(7):3966–3974. [PMC free article] [PubMed] [Google Scholar]
- 14.Li S., Zhang Y., Li S. Risk Factors associated with prolonged mechanical ventilation after corrective surgery for tetralogy of Fallot. Congenit Heart Dis. 2015;10(3):254–262. doi: 10.1111/chd.12205. [DOI] [PubMed] [Google Scholar]
- 15.Li S.J., Zhou K., Shen C. Body surface area: a novel predictor for conversion to thoracotomy in patients undergoing video-assisted thoracoscopic lung cancer lobectomy. J Thorac Dis. 2017;9(8):2383–2396. doi: 10.21037/jtd.2017.07.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Dongen E.I., Glansdorp A.G., Mildner R.J. The influence of perioperative factors on outcomes in children aged less than 18 months after repair of tetralogy of Fallot. J Thorac Cardiovasc Surg. 2003;126(3):703–710. doi: 10.1016/s0022-5223(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 17.Sandoval J.P., Chaturvedi R.R., Benson L. Right ventricular outflow tract stenting in tetralogy of Fallot infants with risk factors for early primary repair. Circ Cardiovasc Interv. 2016;9(12) doi: 10.1161/CIRCINTERVENTIONS.116.003979. [DOI] [PubMed] [Google Scholar]
- 18.Sasikumar D., Sasidharan B., Tharakan J.A. Early and 1-year outcome and predictors of adverse outcome following monocusp pulmonary valve reconstruction for patients with tetralogy of Fallot: a prospective observational study. Ann Pediatr Cardiol. 2014;7(1):5–12. doi: 10.4103/0974-2069.126538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khatib I., Lebret E., Lambert V. Tetralogy of Fallot associated with multiple anomalies. Eur Heart J. 2017;38(4):246. doi: 10.1093/eurheartj/ehw406. [DOI] [PubMed] [Google Scholar]
- 20.Snookes S.H., Gunn J.K., Eldridge B.J. A systematic review of motor and cognitive outcomes after early surgery for congenital heart disease. Pediatrics. 2010;125(4):e818–e827. doi: 10.1542/peds.2009-1959. [DOI] [PubMed] [Google Scholar]
- 21.Di Felice V., Zummo G. Tetralogy of fallot as a model to study cardiac progenitor cell migration and differentiation during heart development. Trends Cardiovasc Med. 2009;19(4):130–135. doi: 10.1016/j.tcm.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 22.Bittel D.C., Butler M.G., Kibiryeva N. Gene expression in cardiac tissues from infants with idiopathic conotruncal defects. BMC Med Genomics. 2011;4:1. doi: 10.1186/1755-8794-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lange M., Kaynak B., Forster U.B. Regulation of muscle development by DPF3, a novel histone acetylation and methylation reader of the BAF chromatin remodeling complex. Genes Dev. 2008;22(17):2370–2384. doi: 10.1101/gad.471408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wydeven N., Posokhova E., Xia Z. RGS6, but not RGS4, is the dominant regulator of G protein signaling (RGS) modulator of the parasympathetic regulation of mouse heart rate. J Biol Chem. 2014;289(4):2440–2449. doi: 10.1074/jbc.M113.520742. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.