Abstract
Identification and treatment of advanced hepatitis C virus (HCV) infection is often challenging. Accurate fibrosis staging can be performed only by liver biopsy. For patients with advanced fibrosis (Metavir score, F3 or F4), progression to decompensated liver disease occurs at a rate of approximately 5% per year and progression to hepatocellular carcinoma occurs at a rate of 1% to 2% per year. Liver decompensation primarily results from altered hepatic blood flow caused by liver scarring and is characterized by ascites and its complications (hepatorenal syndrome, hepatic hydrothorax, and spontaneous bacterial peritonitis), hepatic encephalopathy, bleeding varices, and coagulopathy. Patients with advanced fibrosis need to be regularly monitored for evidence of decompensated disease, and complications need to be aggressively managed. This article summarizes a presentation by Kenneth E. Sherman, MD, at the IAS–USA live continuing medical education course, Management of Hepatitis C Virus in the New Era, held in New York City in April 2011.
Hepatitis C virus (HCV) infection is a curable infectious disease. It is also a liver disease. A critical component of managing HCV-related liver disease is to determine the patient’s severity of liver fibrosis. Patients with advanced fibrosis should be regularly monitored for bleeding varices using esophagogastroduodenoscopy (EGD), and for ascites and hepatocellular carcinoma (HCC) using ultrasound. Complications of liver disease require prompt management and aggressive follow-up. Knowing when to refer patients to a transplant center and doing so in a timely manner is crucial.
Dr Sherman noted that the basic principles in understanding and managing HCV-related liver disease are as follows:
Hepatic fibrosis is not reliably diagnosed by ultrasound examination.
Liver fibrosis rates are not predictable or linear.
Progression from compensated cirrhosis to decompensated liver disease occurs in 5% of patients per year.
Hepatocellular carcinoma develops in 1% to 2% of patients with HCV-related cirrhosis each year.
Hepatitis C Virus–Related Hepatic Fibrosis
Reliable diagnosis of HCV-related advanced fibrosis or cirrhosis depends on liver biopsy (Figure 1). Ultrasound techniques may reveal a shrunken liver or evidence of portal hypertension but do not permit visualization of fibrosis or cirrhosis. Cirrhosis is a histologic diagnosis and is often confused with portal hypertension, which results from cirrhosis. However, not all portal hypertension is caused by cirrhosis. The extent of fibrosis is classified into 4 stages using the Metavir schema. In stage 1, fibrous expansion can be observed in some portal areas. In stage 2, fibrous expansion is observed in most portal areas, with occasional portal-to-portal bridging. Stage 3 fibrosis is characterized by fibrous expansion of portal areas with marked bridging, including portal-to-portal and portal-to-central bridging. Stage 4 fibrosis indicates cirrhosis, which is strictly defined as a liver lobule completely surrounded by scarring.
Figure 1.
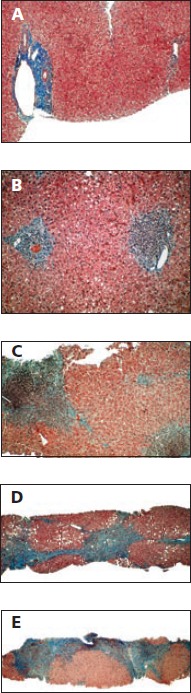
Progression of liver fibrosis as shown on a series of biopsy specimens and as classified by the Metavir schema. A: Liver with no fibrosis. B: Stage 1, fibrous expansion into some portal areas. C: Stage 2, fibrous expansion in most portal areas, with occasional portal-to-portal bridging. D: Stage 3, fibrous expansion of portal areas with marked bridging, including portal-to-portal and portal-to-central bridging. E: Stage 4, cirrhosis. Adapted with permission from Gregory T. Everson, MD, University of Colorado Denver.
Liver fibrosis progression rates are not predictable or linear. They vary widely among patients with HCV infection, with rates being more rapid in those with cofactors for progression that include concurrent HIV infection and excessive consumption of alcohol. Overall, some 15% to 33% of HCV-infected patients may exhibit mild or moderate liver fibrosis over the course of 40 or more years, whereas 20% to 33% have disease that progresses to severe cirrhosis or HCC over 20 or more years. Some patients, however, have liver fibrosis that progresses to severe disease in as little as 3 or 4 years.1
Case: A 48-Year-Old Man with HCV Infection
The patient is a 48-year-old man with HCV infection, a history of 2 years of injection drug use from age 19 years to 21 years, and moderate social alcohol use. The patient presents with fatigue and insomnia, with laboratory evaluation showing alanine aminotransferase (ALT) level of 89 U/L, aspartate aminotransferase (AST) level of 67 U/L, bilirubin level of 1.5 mg/dL, alkaline phosphatase level of 197 IU/L, hemoglobin level of 13.4 gm/dL, platelet count of 111,000/μL, HCV RNA level of 2.7 million IU/mL, and HCV genotype 1b. Right upper quadrant ultrasound shows an echogenic liver consistent with fat or liver disease. Instead of undergoing biopsy for staging of fibrosis, the patient is started on a 3-drug regimen containing pegylated interferon alfa, ribavirin, and a direct-acting antiviral.
After 12 weeks of treatment, virus is undetectable. The patient notes a 10-lb weight gain, and his wife complains that he sometimes appears drunk, though he denies alcohol use. What is the best next step in the treatment of this patient: 1. Prescribe an antidepressant? 2. Reassure the patient that he is experiencing common side effects of interferon alfa therapy? 3. Order a stat ultrasound? 4. Suggest diet and exercise?
Dr Sherman explained that an ultrasound will determine if the weight gain is associated with the development of ascites, and is thus the best next step. Although the severity of fibrosis in this patient is unknown, his workup prior to treatment initiation produced some findings that raise concern over the potential for decompensated liver disease, including a somewhat low platelet level and the presence of fatigue and insomnia. Reversal of sleep patterns is a common very early symptom of hepatic encephalopathy.
Weight gain is not expected in patients receiving interferon alfa; in fact, interferon alfa treatment is more commonly associated with anorexia and weight loss. Ultrasound reveals that the patient has ascites, indicating the presence of advanced liver disease that became decompensated as a result of anti-HCV therapy.
In a sense, there are 2 timelines to keep in mind when considering progression of HCV-related liver disease. One timeline begins when a patient becomes infected with HCV, marking the onset of liver injury and a course of progression (in the absence of successful treatment) that varies from several years to as long as 50 years. The second timeline begins at the onset of cirrhosis. Progression from compensated asymptomatic cirrhosis to decompensated liver disease occurs in approximately 5% of HCV-infected patients per year. In addition, HCC develops in 1% to 2% of patients with HCV-related cirrhosis per year and eventually results in symptomatic decompensated disease.
Cirrhosis and Hepatic Decompensation
Approximately 5% of HCV-infected patients with cirrhosis will have progression to hepatic decompensation per year. Hepatic decompensation is characterized by: (1) ascites, which itself may be complicated by hepatorenal syndrome (HRS), hepatic hydrothorax, and spontaneous bacterial peritonitis (SBP); (2) encephalopathy; (3) bleeding varices; and (4) coagulopathy (indicated by a prothrombin time [PT] 3 seconds above the control time or by an international normalized ratio [INR] greater than 1.5). Clinical staging of cirrhosis was traditionally performed using Child-Turcotte-Pugh scoring, a scoring system initially developed to predict a patient’s survival associated with portal shunt surgery. It was later adapted to determine priority for liver transplantation. Scores are based on presence and degree of abnormalities in bilirubin level, albumin level, INR, and on presence and severity of hepatic encephalopathy and ascites. Cirrhosis staging under this system is divided into class A, B, or C, with each class having an assigned 1- and 2-year survival rate.
The Child-Turcotte-Pugh scoring system is not highly reliable for predicting mortality, however, and in recent years has been replaced by the more complex model for end-stage liver disease (MELD) scoring system. MELD scoring uses bilirubin, creatinine levels, and the INR to predict mortality risk and determine timing of orthotopic liver transplantation. For example, for a patient with end-stage liver disease, a creatinine level of 1.6 mg/dL, bilirubin level of 1.4 mg/dL, and an INR of 1.6, the MELD system will predict a 3-month mortality risk of 18%.
Most of the damage in decompensated liver disease is related to alteration in blood flow through the liver. The portal vein, which supplies the blood to the liver, is formed by the confluence of the superior and inferior mesenteric veins and the splenic vein (Figure 2). A buildup of fibrotic scar tissue in the liver can cause obstruction of blood flow into the liver, causing blood to back up in the many vessels in the splanchnic circulation and resulting in numerous adverse consequences. The spleen becomes enlarged and begins to filter nonsenescent blood cells from the circulation, resulting in decreased levels of platelets, red blood cells, and white blood cells. Decreased absorption of fluids along the peritoneal surface also occurs because lymphatic drainage is supplemented by blood flow from the peritoneal space into the splanchnic circulation, resulting in ascites.
Figure 2.
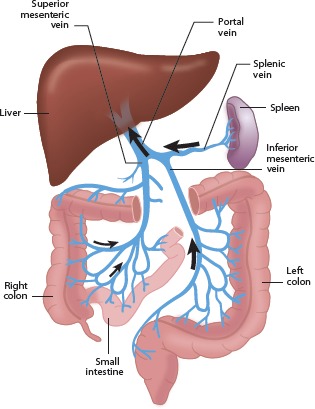
Splanchnic circulation. Arrows indicate direction of blood flow.
A response to reduced blood flow into the liver is the development of collateral vessels. These vessels grow mostly in mucosal junction areas and can result in the development of varices in the esophagus, stomach, and rectum. Recanalization of vessels at the umbilical vein occurs in some patients, driving the development of new vessels across the anterior abdomen (called caput medusae).
Management of Ascites and its Complications
Ascites can cause substantial pain and discomfort. Patients may ask for narcotics for pain relief, but narcotic use should be avoided if possible by patients who already have hepatic impairment. As noted, complications of ascites include HRS, hepatic hydrothorax, and SBP. Large ascites should also be relieved at first observation by tapping (also known as large volume paracentesis [LVP]). Every time the patient is admitted to the hospital and with every change in health status (eg, worsening ascites, development of bleeding varices, or worsening hepatic encephalopathy), a diagnostic tap should be performed. Tapping at the time of hospital admission is important because a high proportion of patients with ascites have SBP at the time of admission, whether or not fever or abdominal pain is present, and SBP is associated with marked morbidity and mortality.
Tapping does not require that the patient be sent to interventional radiology for a computed tomography (CT)–guided tap. The procedure is safely performed in the midline subumbilical area with the patient sitting at a 30-degree angle. For a diagnostic tap, the procedure can be accomplished in any patient with a 1.5-inch needle (and with a 1.0-inch needle in most). Tapping is very safe regardless of the patient’s degree of coagulopathy, as the area beginning approximately 2 cm below the umbilicus is relatively avascular. There is no need to have fresh frozen plasma or platelets on hand for the procedure. Cell counts on the fluid should be performed and fluid should be injected directly into bedside culture bottles, because false-negative results occur in 40% to 50% of cases in which the fluid is processed in the laboratory rather than inoculated at bedside. For therapeutic taps, a large-gauge multi-perforated Caldwell or similar needle is used. Albumin replacement is required if the patient’s creatinine level is elevated or if more than 5 L of fluid is removed.
Management of ascites is diagrammed in Figure 3. At the initial visit for patients with tense ascites, a large-volume tap is performed to relieve discomfort as quickly as possible. Patients are placed on a sodium-restricted diet (maximum of 2 g/day) and diuretic therapy is initiated. The most effective approach in diuretic treatment of ascites is not to start with higher doses of the rapid-acting agent furosemide, but rather to start with slower-acting treatment such as the aldosterone antagonist spironolactone (50 mg/day) in combination with lower doses of furosemide (20 mg/day or 40 mg/day) or bumetanide (1 mg/day); doses are then titrated upward at 2-week intervals to maximum doses of 400 mg/day for spironolactone with 160 mg/day for furosemide, or 4 mg/day for bumetanide.
Figure 3.
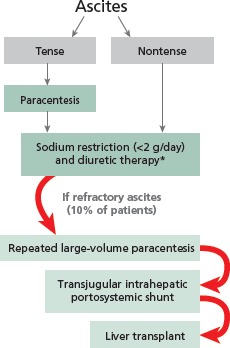
Schematic representation of the management of ascites. *For diuretic therapy, initiate treatment with spironolactone (50 mg/day) in combination with furosemide (20 mg/day or 40 mg/day) or bumetanide (1 mg/day). Titrate stepwise at 2-week intervals to spironolactone 400 mg/day, furosemide 160 mg/day, or bumetanide 4 mg/day, as long as regimen is tolerated.
When ascites is managed appropriately, the risk of progressing to refractory ascites is relatively low. When refractory ascites does occur, it is treated with repeated large-volume paracentesis. Consideration should be given to placement of a transjugular intrahepatic portosystemic shunt (TIPSS), which is successful in reducing ascites in 60% to 70% of cases and eliminating it in 20% to 30% of cases. Placement of TIPSS should only be performed by highly experienced interventional radiologists after evaluation of the patient by a hepatologist. Finally, the patient’s candidacy for liver transplantation should be considered.
Spontaneous Bacterial Peritonitis
SBP is diagnosed by examining the ascitic fluid: greater than 500 white blood cells/mL, greater than 250 polymorphonuclear cells/mL, or a positive culture result are indicative of SBP. Each definition listed is trumped by the next one on the list. Treatment is with a 5-day course of intravenous cefotaxime or other cephalosporin. For beta-lactam allergy, ciprofloxacin may be used. Repeat paracentesis should be considered at 48 hours to 72 hours after treatment initiation to check for white blood cell response; absence of response may suggest the presence of an organism with drug resistance or a complex polymicrobial process that is not SBP. Albumin infusions should be given to patients with SBP, as they have been shown to reduce mortality.
One study showed a reduction in 60-day mortality from 29% with cefotaxime alone to 10% with cefotaxime plus 1.5 g/kg albumin administered within 6 hours of SBP diagnosis, followed by 1.0 g/kg on day 3.2 Antibiotic prophylaxis with ciprofloxacin (750 mg/week) has been shown to prevent SBP in patients with ascites, with one classic study showing a 6-month incidence of SBP of 4% with ciprofloxacin versus 22% with placebo.3 Alternative antibiotics for prophylaxis are norfloxacin and trimethoprim-sulfamethoxazole.
Hepatic Hydrothorax
The ascites complication of hepatic hydrothorax results from a break in the diaphragm that creates a ball-valve effect such that each breath causes inflow of fluid from the abdomen into the pleural space. The right pleural space is much more frequently involved than the left. Hepatic hydrothorax is not to be confused with primary pleural effusion, and it is not to be treated with insertion of a chest tube. Placement of a chest tube in a patient with hepatic hydrothorax results in a continuous loss of protein that rapidly renders the patient nutritionally depleted, making liver transplantation impossible. Patients with hepatic hydrothorax may or may not have visible ascites.
Hepatic hydrothorax should be suspected for any patient with advanced liver disease and right-sided effusion. Hepatic hydrothorax should be managed by aggressive diuresis, pleural taps when needed, and immediate consideration for liver transplantation. Patients requiring frequent pleural taps may gain additional MELD points on the transplant list.
Varices
Patients with cirrhosis must be monitored for the development of varices (Figure 4) and variceal bleeding using EGD. Those with no varices should have repeat EGD performed at 3 years for well-compensated liver disease and at 1 year for decompensated disease. Patients with small varices (Grade 1) may begin nonselective beta-blocker prophylaxis. Patients with medium (Grade 2, 5-10 mm) or large (Grade 3,>10 mm) varices who have no red wales (evidence of impending bleeding) should receive beta-blocker treatment; those with red wales should be given beta-blocker treatment and considered for esophageal band ligation. Esophageal varices develop in 35% to 80% of patients with cirrhosis, and bleeding occurs in 25% to 40% of those with varices. Of those with bleeding, 30% to 50% die within 90 days. Of the 50% to 70% who survive an initial bleeding episode, 70% will experience subsequent bleeding.
Figure 4.
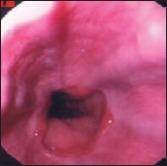
Gastroscopy image of esophageal varices.
Hepatic Encephalopathy
Hepatic encephalopathy is classically detected by the observation of hand asterixis (also known as liver flapping or flapping tremor). It is caused by the shunting of gut-derived neuroactive substances that cross the blood-brain barrier; these substances are mostly nitrogen based and act as inhibitory neurotransmitters. Survival probabilities for patients with hepatic encephalopathy are 42% at 1 year and 23% at 3 years. Precipitating factors for hepatic encephalopathy include a high-protein diet, gastrointestinal bleeding, infection (including SBP), vascular thrombosis, HCC, and poor adherence to hepatic encephalopathy treatment. Traditional treatments for hepatic encephalopathy include the use of lactulose or other nonabsorbable sugars, which cause osmotic diarrhea and movement of nitrogen compounds out of the gut, and the use of antibiotics such as neomycin and metronidazole that change the gut flora.
A recent phase III trial showed that, compared with placebo, the nonabsorbable antibiotic rifaximin administered at 550 mg twice daily was associated with a statistically significant reduction in episodes of breakthrough hepatic encephalopathy in patients in remission from recurrent hepatic encephalopathy (breakthrough episodes occurred in 22.1% of patients taking rifaximin vs 45.9% with placebo; P < .001).4 More than 90% of patients in the trial received concomitant lactulose therapy. Rifaximin treatment was also associated with a statistically significant reduction in frequency of hospitalization for hepatic encephalopathy (13.6% hospitalization rate with rifaximin vs 22.6% with placebo, P = .01). The incidence of overall and serious adverse events was similar in the rifaximin and placebo recipients.
Liver Transplantation
Criteria for referring patients for liver transplantation include any signs of hepatic decompensation (eg, ascites, hepatic encephalopathy, or variceal bleeding), a MELD score greater than 10, or HCC at any level of MELD. Unfortunately, the need for liver transplantation outstrips the availability of liver donors. In the United States, only about 5500 livers become available each year. Referring patients for transplantation expeditiously can be lifesaving. Encouraging people to become donors is lifesaving at the community level.
References
- 1.Afdhal NH. The natural history of hepatitis C. Semin Liver Dis. 2004;24(Suppl 2): 3-8. [DOI] [PubMed] [Google Scholar]
- 2.Sort P, Navasa M, Arroyo V, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999;341:403-409. [DOI] [PubMed] [Google Scholar]
- 3.Rolachon A, Cordier L, Bacq Y, et al. Ciprofloxacin and long-term prevention of spontaneous bacterial peritonitis: results of a prospective controlled trial. Hepatology. 1995;22:1171-1174. [DOI] [PubMed] [Google Scholar]
- 4.Bass NM, Mullen KD, Sanyal A, et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362:1071-1081. [DOI] [PubMed] [Google Scholar]


