Abstract
Acute kidney injury (AKI) and chronic kidney disease (CKD) are more common in HIV-infected persons than in the general population. AKI is associated with poor health outcomes, including increased risk of heart failure, cardiovascular events, end-stage renal disease (ESRD), and mortality. The most common causes of AKI in HIV-infected persons are systemic infections and adverse drug effects. The prevalence of CKD is rising in the HIV-infected population and CKD is increasingly likely to be caused by comorbid conditions, such as diabetes and hypertension, that frequently cause CKD in the general population. Guidelines for CKD screening in HIV-infected patients are being revised. It is currently recommended that all patients be screened for creatinine-based estimates of glomerular filtration rate and for urine protein at the time of HIV diagnosis. Annual screening is recommended for high-risk patients. Hemodialysis, peritoneal dialysis, and kidney transplantation are all options for treating ESRD in HIV-infected patients. Hemodialysis and peritoneal dialysis offer similar survival in HIV-infected patients with ESRD. In selected patients with well-controlled HIV infection, kidney transplantation is associated with survival intermediate between that in the overall transplant population and that among transplant recipients older than 65 years. This article summarizes a presentation by Christina M. Wyatt, MD, at the IAS–USA continuing medical education program held in Chicago in May 2012, describing AKI and CKD using case illustrations.
HIV-associated nephropathy (HIVAN) is not the only cause of kidney disease in patients with HIV infection. Acute kidney injury (AKI) is more common in HIV-infected persons than in the general population and is associated with poor health outcomes. The prevalence of chronic kidney disease (CKD) is also increasing in the HIV-infected population, with a growing burden of CKD related to comorbid diabetes and hypertension. Dr Wyatt presented a series of cases to illustrate the issues of kidney disease in HIV infection.
Acute Kidney Injury
Case Illustration 1
A 56-year-old African-American woman presents with a 2-week history of nausea and vomiting. She has a history of AIDS, with a last measured CD4+ cell count of approximately 300/μL and well compensated cirrhosis due to hepatitis C virus (HCV) infection. Her antiretroviral medication consists of tenofovir/emtricitabine and ritonavir-boosted lopinavir. She has been taking ibuprofen for the past week for general malaise. She had missed her most recent follow-up visit with her physician. Her laboratory evaluation shows a serum creatinine level of 21 mg/dL, up from the prior measurement of 1.4 mg/dL. Apart from acidosis, routine electrolytes including sodium, chloride, potassium, and glucose are unremarkable. Urinalysis shows elevated protein, ketones, and glucose. An x-ray taken to rule out gastrointestinal obstruction is normal.
Characteristics of AKI. As noted, AKI is more common in HIV-infected individuals than in the noninfected general population. Several studies have indicated that risk factors for acute injury include underlying CKD, advanced HIV disease (whether measured by CD4+ cell count or HIV viral load), and HCV coinfection.1-3 AKI is predictive of poor health outcomes in HIV-infected patients as well as in the general population, with even an asymptomatic increase in serum creatinine being associated with increased risk of heart failure, cardiovascular events, end-stage renal disease (ESRD), and death (Figure 1).2,4
Figure 1.
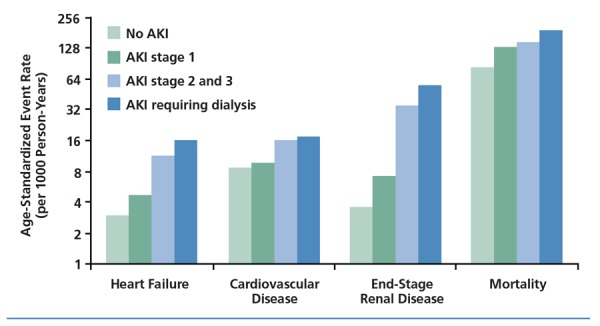
Outcomes in HIV-infected patients with no acute kidney injury (AKI) or AKI of increasing severity. Adapted from Choi et al.4
In a cohort of approximately 750 patients followed prospectively at a single institution,1 more than 10% developed at least 1 episode of AKI. Most of these cases required hospitalization or occurred as part of a concurrent illness and hospitalization. In 52% of cases, the injuries were attributed to systemic infections, with 76% of these being AIDS-defining infections. These cases usually presented as a prerenal disorder or acute tubular necrosis (ATN).
Drug treatment was identified as the cause of acute injury in 32% of cases, with implicated drugs including antibiotics (eg, beta-lactams and aminoglycosides), indinavir or tenofovir, radiocontrast agents, nonsteroidal antiinflammatory drugs (NSAIDs), and lithium. The clinical presentation was variable, including ATN, interstitial nephritis, crystalluria/obstruction, or prerenal presentations associated with gastrointestinal effects of illness. Liver failure accounted for 10% of cases, with 90% of these being attributed to HCV disease.
Case Illustration 1, continued
Subsequent laboratory evaluation showed that the patient had a phosphorus level of 5.2 mg/dL and urine sodium level of 60 mEq/L. Among the potential causes of AKI that should be considered in this patient are prerenal effects, hepatorenal syndrome in association with cirrhosis, and tenofovir toxicity. Postrenal causes are unlikely, since there is no evidence of obstruction. Diabetic ketoacidosis generally needs to be considered in the differential diagnosis of AKI with glycosuria, but is unlikely in this patient because she has no history of diabetes and has a normal blood glucose level. In fact, the only reason a patient with normal serum glucose should have glucose in the urine is tubular dysfunction. Whether or not a patient is diabetic, the presence of “euglycemic” glycosuria is a sign of proximal tubular injury—and this is a typical presentation of tenofovir toxicity.
Tenofovir toxicity. The typical presentation of tenofovir toxicity is proximal tubulopathy. Approximately 2% of patients taking tenofovir develop substantial toxicity, with subclinical abnormalities being more frequent. Numerous studies have shown small reductions in estimated creatinine clearance or glomerular filtration rate (eGFR) in tenofovir recipients, with one meta-analysis indicating a mean difference of 3.9 mL/min in estimated creatinine clearance rate between patients receiving tenofovir and patients not receiving tenofovir (Figure 2).5 The clinical relevance of these findings is unclear, and to date there have been no tenofovir-specific studies examining the implications of subclinical abnormalities for longer-term outcomes. A 2010 report from the EuroSIDA cohort indicated that cumulative exposure (up to more than 3 years) to tenofovir, indinavir, and ritonavir-boosted lopinavir was associated with a small but statistically significant increase (P < .0001 for each drug) in risk of CKD, defined as an eGFR of less than 60 mL/min.6 A higher risk of CKD was associated with cumulative exposure to atazanavir (P < .0001), although the magnitude of increased risk (from 1 case per 100 patient-years at the start of treatment to 4 cases per 100 patient-years at more than 3 years of follow-up) observed in the EuroSIDA cohort has not been seen in other studies.
Figure 2.
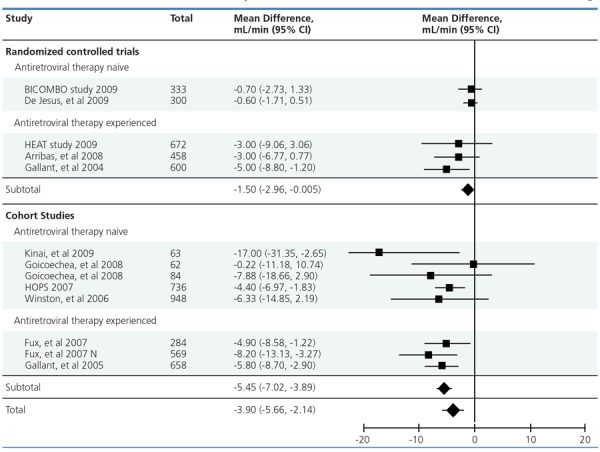
Mean difference and 95% confidence interval (CI) in estimated creatinine clearance rate between patients receiving tenofovir and patients not receiving tenofovir in selected randomized controlled trials and cohort studies. Adapted from Cooper et al.5
In a recent retrospective study conducted by the Veterans Affairs Medical Center in San Francisco, cumulative exposure to tenofovir was found to be statistically significantly associated with increased risk of CKD (defined as eGFR less than 60 mL/min or more rapid decline in eGFR) in every patient subgroup examined, except for patients with diabetes and patients with preexisting CKD (Figure 3).7 The hazard ratios for the subgroups did not exceed 1.51 and generally were in the range of 1.2 to 1.4, representing a relatively small increase in risk over a low baseline risk. The findings of this study have sometimes been misinterpreted by patients as showing absolute risk of kidney dysfunction (ie, the hazard ratios of 1.2 to 1.4 have been misinterpreted as indicating an absolute risk of 20% to 40%).
Figure 3.
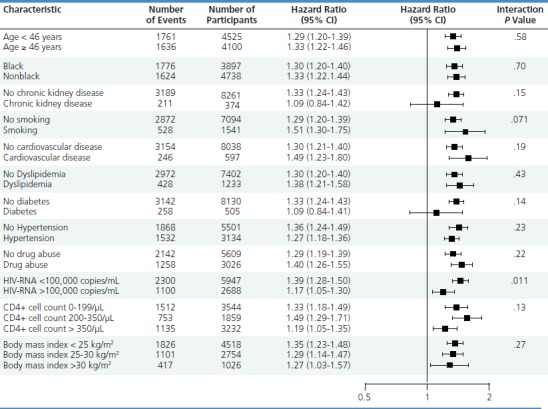
Hazard ratios and 95% confidence intervals (CI) for chronic kidney disease (defined as glomerular filtration rate [GFR] of less than 60 mL/min or more rapid decline in GFR) according to subgroups of patients receiving tenofovir. P value is for interaction within subgroups. Adapted from Scherzer et al.7
The risk factors for tenofovir toxicity are not well defined, but likely include unrecognized low GFR or reduced GFR associated with concomitant conditions. In addition, ritonavir-boosted protease inhibitors (PIs) have been associated with an increased risk of tenofovir toxicity. Some initial data suggested that tenofovir renal toxicity may be associated with single nucleotide polymorphisms in renal transporters, although such findings have not been confirmed in larger studies. Given the association of tenofovir renal toxicity with concomitant use of ritonavir-boosted PIs, initial studies focused on alterations in the proximal tubular transporter gene multidrug resistance-associated protein 2 (MRP2), which is known to be inhibited by ritonavir. However, tenofovir is trafficked from proximal tubular cells by a different transporter, multidrug resistance-associated protein 4 (MRP4), and it remains unclear how genetic alterations in MRP2 would affect tenofovir transport. It has also been thought that the decline in GFR observed with tenofovir may be the result of inhibition of tubular secretion of creatinine, similar to what has been observed with trimethoprim-sulfamethoxazole and what has been suggested to occur with the investigational antiretroviral-boosting agent cobicistat. However, available data indicate that such inhibition does not occur with tenofovir.
With regard to other issues involving tenofovir renal toxicity, tenofovir is used to treat hepatitis B virus (HBV) infection and to date there is no signal of renal toxicity from trials in HIV-uninfected HBV-infected patients. However, it bears noting that initial trials of tenofovir in HIV-infected patients also provided little indication of potential renal toxicity. Tenofovir is also used in HIV preexposure prophylaxis (PrEP), and to date there is no evidence of significant renal toxicity in HIV-uninfected individuals receiving tenofovir-containing PrEP. There was a nonsignificant trend toward increased creatinine levels in subjects from the iPrEx (Chemoprophylaxis for HIV Prevention in Men) study of tenofovir/emtricitabine in PrEP (2% versus 1% in placebo-treated patients, P =0.08), but no difference in serum creatinine or phosphorus abnormalities in the Partners PrEP study.
There is interest in performing a pooled analysis of potential tenofovir renal toxicity in numerous PrEP study populations. Use of an investigational fixed-dose pill containing tenofovir/emtricitabine, the HIV integrase strand transfer inhibitor elvitegravir, and the elvitegravir-boosting agent cobicistat results in a decrease in eGFR compared with fixed-dose tenofovir/emtricitabine/efavirenz. Cobicistat is associated with a rapid and reversible decrease in estimated GFR, but no change in measured GFR, because it interferes with creatinine secretion. Although there is no evidence that cobicistat is nephrotoxic, use of tenofovir and cobicistat together may complicate the diagnosis of tenofovir renal toxicity. Whether the tenofovir prodrug in development poses a decreased risk of renal toxicity compared with tenofovir disoproxil fumarate also remains to be seen.
Chronic Kidney Disease
Case Illustration 2
A 43-year-old African-American woman presents with stage 5 CKD. She has HIV and HCV coinfection, with a nadir CD4+ cell count of more than 200/μL. She has had hypertension for 20 years and type 2 diabetes for 8 years and has a body mass index of 31 kg/m2. She currently is receiving, at her own choice, suboptimal antiretroviral therapy with zidovudine and lamivudine, yet her viral load has remained below detection limits and her CD4+ cell count is currently 598/μL. She receives amlodipine and lisinopril for hypertension and insulin for diabetes, but blood pressure and blood glucose are poorly controlled. Her blood pressure is 156/98 mm Hg, serum creatinine level is 6.2 mg/dL, and serum phosphorus value is 6.4 mg/dL. Urinalysis shows 3+ proteinuria and 1+ glycosuria, and the urine:creatinine ratio is 3.2, indicating approximately 3 grams of proteinuria/24 hours.
Potential CKD diagnoses in this patient include diabetic nephropathy, hypertensive nephrosclerosis, and HCV-related glomerulonephritis, and further workup is necessary to arrive at a definitive diagnosis. It is unlikely that the patient has HIVAN. African-American patients have excess risk of HIVAN, which has been linked to single nucleotide polymorphisms on chromosome 22, although debate continues on which gene or genes are affected. However, HIVAN is classically associated with advanced HIV disease, and the patient has undetectable viral load and an elevated CD4+ cell count.
Changing spectrum of CKD. Data published in 2004 indicated that nearly half of cases of CKD in HIV-infected patients were caused by HIVAN. Smaller, roughly equal proportions of CKD were caused by immune complex disease, membranous/membranoproliferative glomerulonephritis in association with viral hepatitis, and diabetes or hypertension. An even smaller proportion was caused by acute interstitial nephritis.8 However, studies since then suggest that the spectrum of CKD in HIV-infected patients is changing with less HIVAN and more comorbid kidney disease, such as that caused by hypertension and diabetes.9
Dr Wyatt believes that if all HIV-infected patients with CKD underwent renal biopsy, results would show that diabetes and hypertension are the leading causes of the disease, as they are in the general population. Indeed, kidney biopsy is underused for diagnosis, and would likely clarify the diagnosis in the patient in this case illustration. There is a longstanding perception that HIV-infected patients are at increased risk for complications of kidney biopsy; however, a large retrospective case series from The Johns Hopkins University School of Medicine did not demonstrate any increased risk of complications in HIV-infected individuals, apart from a small increase in risk in those coinfected with HCV.10
Guidelines for CKD screening in HIV-infected patients are in the process of being revised. It is currently recommended that all patients be screened for creatinine-based eGFR and urine protein at the time of HIV diagnosis. Annual screening is recommended in high-risk patients, including African-American patients and those with HCV coinfection, advanced HIV disease, diabetes, or hypertension.
Case Illustration 2, continued
The patient agreed to a kidney biopsy, because she was considering changing her antiretroviral regimen and it was agreed that she would do so if HIVAN was demonstrated on biopsy. The biopsy showed advanced diabetic nephropathy and hypertensive vascular changes. There is no reason to believe that management of the CKD in this patient should differ from management of CKD from these causes in the general population. Thus, management should include tight blood pressure and glycemic control, weight loss, and cardiovascular risk modification, as well as a nephrology referral. Improved blood pressure and glycemic control are effective in delaying progression of CKD in the general population. Cardiovascular risk modification, including weight loss and smoking cessation when necessary, are important not only because they might improve the natural history of the kidney disease, but because patients with CKD are at increased cardiovascular risk. Drug regimens and dosing should also be reviewed. Apart from any need for diagnostic testing, referral to a nephrologist is appropriate for ESRD planning. In ESRD planning, hemodialysis, peritoneal dialysis, and kidney transplantation should be discussed with the patient.
HIV and ESRD. Data on prevalence of ESRD in HIV-infected patients are limited to ESRD caused by HIVAN. These data show an increasing prevalence of ESRD in the HIV-infected population, despite stabilization of the incidence of HIVAN-related ESRD that has occurred with wide use of antiretroviral therapy. It is likely that the prevalence of ESRD from any cause has also increased among HIV-infected patients. The prevalence of HIV infection in dialysis units is variable, with higher prevalence in urban centers such as New York City and Baltimore.
Hemodialysis, peritoneal dialysis, and kidney transplantation are all options for managing ESRD in HIV-infected patients. Survival rates are very similar with hemodialysis and peritoneal dialysis in HIV-infected patients with ESRD. Thus, it is reasonable to offer both options to patients and let them decide based on quality-of-life issues. Many patients have a strong preference for one modality or the other. For patients in whom hemodialysis is planned, early referral for fistula creation is essential to avoid use of a tunneled catheter. Development of a functioning fistula requires at least 8 weeks and may take up to 6 months in some patients. For patients on dialysis, antiretroviral drug regimens and doses should be carefully reviewed and adjusted if necessary.
A National Institutes of Health–sponsored study assessed outcomes of kidney transplantation in 150 HIV-infected patients with undetectable viral load, CD4+ cell count greater than 200/μL, and stable antiretroviral therapy.11 Patient and graft survival rates were acceptable, being somewhat poorer than rates in the overall transplant population and somewhat better than those among HIV-uninfected transplant recipients older than 65 years. No increase in frequency of opportunistic infections was observed, and the 5 AIDS-defining illnesses that occurred can also be observed in HIV-uninfected kidney transplant recipients. However, substantial drug interactions occurred, particularly with PIs and nonnucleoside analogue reverse transcriptase inhibitors. It is crucial that any potential changes in antiretroviral regimens in the posttransplantation period be discussed with patients’ transplant team. There can be large swings in trough levels of tacrolimus or cyclosporine, the calcineurin inhibitors used for immunosuppression, with changes in antiretroviral regimen, and some patients have lost their grafts due to changes in antiretroviral regimens in the posttransplantation period.
It should be noted that the patient under discussion likely would not be considered a candidate for transplantation unless her antiretroviral regimen were optimized and it was found that she could tolerate a stable optimal regimen.
Summary
AKI is common in HIV-infected patients and is associated with poor outcomes. Antiretroviral nephrotoxicity may be difficult to distinguish from AKI or CKD from other causes. Comorbid CKD is increasingly prevalent in HIV-infected patients. HIV-infected patients are candidates for hemodialysis or peritoneal dialysis, and select patients may be candidates for kidney transplantation.
References
- 1.Franceschini N, Napravnik S, Eron J, Jr., Szczech LA, Finn WF. Incidence and etiology of acute renal failure among ambulatory HIV-infected patients. Kidney Int. 2005;67(4):1526-1531. [DOI] [PubMed] [Google Scholar]
- 2.Wyatt CM, Arons RR, Klotman PE, Klotman ME. Acute renal failure in hospitalized patients with HIV: risk factors and impact on in-hospital mortality. AIDS. 2006;20(4):561-565. [DOI] [PubMed] [Google Scholar]
- 3.Roe J, Campbell LJ, Ibrahim F, Hendry BM, Post FA. HIV care and the incidence of acute renal failure. Clin Infect Dis. 2008;47(2):242-249. [DOI] [PubMed] [Google Scholar]
- 4.Choi AI, Li Y, Parikh C, Volberding PA, Shlipak MG. Long-term clinical consequences of acute kidney injury in the HIV-infected. Kidney Int. 2010;78(5):478-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper RD, Wiebe N, Smith N, Keiser P, Naicker S, Tonelli M. Systematic review and meta-analysis: renal safety of tenofovir disoproxil fumarate in HIV-infected patients. Clin Infect Dis. 2010;51(5):496-505. [DOI] [PubMed] [Google Scholar]
- 6.Mocroft A, Kirk O, Reiss P, et al. Estimated glomerular filtration rate, chronic kidney disease and antiretroviral drug use in HIV-positive patients. AIDS. 2010;24(11):1667-1678. [DOI] [PubMed] [Google Scholar]
- 7.Scherzer R, Estrella M, Li Y, Deeks SG, Grunfeld C, Shlipak MG. Association of tenofovir exposure with kidney disease risk in HIV infection. AIDS. 2012;26(7):867-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Szczech LA, Gupta SK, Habash R, et al. The clinical epidemiology and course of the spectrum of renal diseases associated with HIV infection. Kidney Int. 2004;66(3):1145-1152. [DOI] [PubMed] [Google Scholar]
- 9.Berliner AR, Fine DM, Lucas GM, et al. Observations on a cohort of HIV-infected patients undergoing native renal biopsy. Am J Nephrol. 2008;28(3):478-486. [DOI] [PubMed] [Google Scholar]
- 10.Tabatabai S, Sperati CJ, Atta MG, et al. Predictors of complication after percutaneous ultrasound-guided kidney biopsy in HIV-infected individuals: possible role of hepatitis C and HIV co-infection. Clin J Am Soc Nephrol. 2009;4(11):1766-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stock PG, Barin B, Murphy B, et al. Outcomes of kidney transplantation in HIV-infected recipients. N Engl J Med. 2010;363(21):2004-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]


