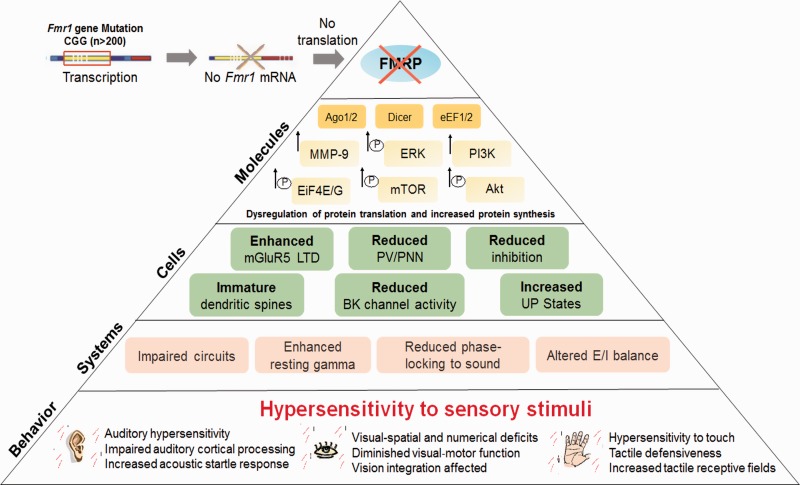
Figure 1.
Mechanisms of sensory phenotypes associated with FXS. Fragile X syndrome is associated with an expansion of CGG repeats in 5′ untranslated area of the fragile X mental retardation 1 (Fmr1) gene, which leads to silencing Fmr1 gene and a partial or full loss of the fragile X mental retardation protein (FMRP). FMRP is an RNA-binding protein that regulates translation of mRNAs at synapses, some of which encode proteins involved in protein synthesis and synaptic plasticity. FMRP is known to regulate protein translation through eukaryotic translation elongation factor 1/2 (eEF1/2), argonaute proteins (Ago1/2), eukaryotic translation initiation factor 4 E/G (eIF4E/G), and Dicer. FMRP may also directly regulate phosphatidylinositide-3-kinase (PI3K), Akt, mammalian target of rapamycin (mTOR), and extracellular signal-regulated kinase (ERK) signaling. Lack of FMRP also leads to enhanced metabotropic glutamate receptor 5 (mGluR5)-mediated long-term depression (LTD), reduced voltage and Ca2+ activated K+ (BK) channel activity, and increased matrix metalloproteinase-9 (MMP-9) activity, which affect cellular responses resulting in reduced inhibition, impaired development of parvalbumin (PV) interneurons and perineuronal nets (PNN), increased UP states, and abnormal dendritic spine development. These molecular and cellular alterations can contribute to system-level changes, such as impaired development of neural circuits, enhanced resting gamma, and altered excitatory/inhibitory (E/I) balance, which may underlie sensory hypersensitivity and altered behaviors observed in FXS.