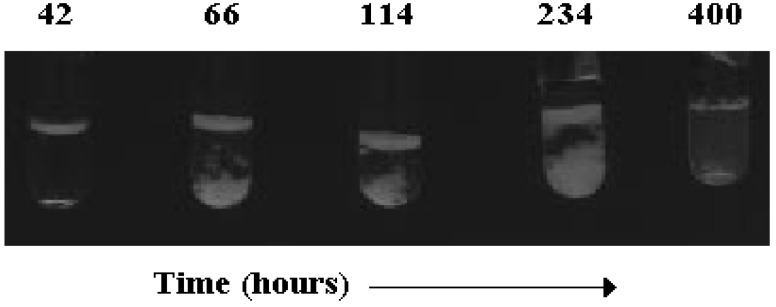
Figure 5.
Phase evolution with time, in hours, in a solution containing 41.5 wt% sodium taurodeoxycholate, NaTDC, viewed between crossed polarisers. The solution was formerly heated and equilibrated at 25.0 °C. Note the presence of an upper anisotropic layer, changing its thickness and consistency with time (lanes 2-4), the formation of small crystals in the bottom (lanes 2 and 3), creaming (lane 4), and formation of a birefringent, liquid crystal (lane 5).