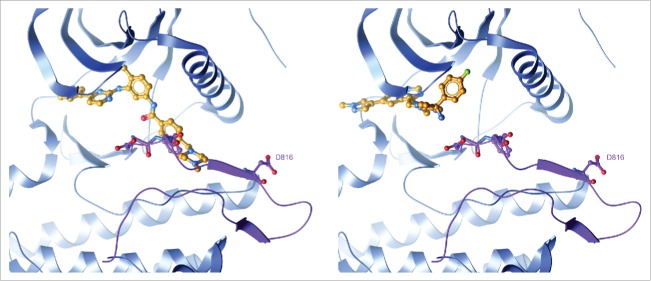
Figure 1.
Avapritinib, a type I inhibitor, specifically binds the active conformation of the KIT kinase domain. Left: Structure of imatinib bound to KIT proto-oncogene receptor tyrosine kinase (KIT) with an open activation loop conformation. The open activation loop was modelled by superimposing the active, KIT:adenosine diphosphate (ADP) crystal structure (PDB code 1PKG) on the inactive, KIT:imatinib crystal structure (PDB code 1T46) and replacing the activation loop segment of 1T46 (residues 809–834) with that of 1PKG. The activation loop is colored purple with the Asp-Phe-Gly (DFG) motif and residue D816 shown in ball-and-stick. Steric clash with Phe811 highlights the inability of type II inhibitors like imatinib to bind to an active kinase conformation. Right: Avapritinib docked into the active 1T46/1PKG hybrid structure. Avapritinib readily fits into the adenosine 5′-triphosphate (ATP)-binding pocket with the activation loop in an open, DFG-in conformation.