Figure 1.
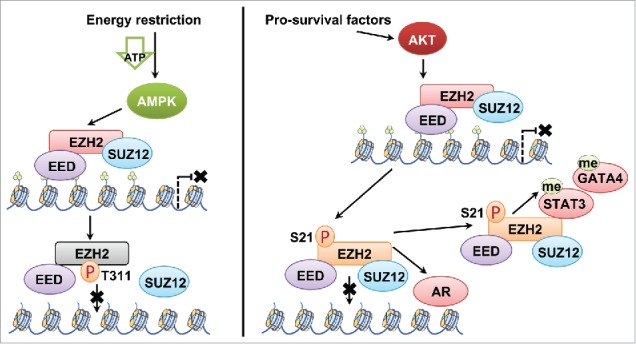
Distinct mechanisms of AMPK and Akt in controlling PRC2 activity. AMPK phosphorylates Thr311-EZH2, which disrupts the interaction between SUZ12 and EZH2 to inhibit the PRC2 holoenzyme activity. Like pS21-EZH2, pT311-EZH2 results in reduced H3K27 tri-methylation. However, unlike pS21-EZH2, pT311-EZH2 disrupts the integrity of the PRC2 holoenzyme, thereby mainly serves to inhibit the oncogenic role of PRC2. On the other hand, Akt phosphorylates Ser21-EZH2 to disrupt the interaction between PRC2 and the H3 tail, leading to reduced H3K27 tri-methylation. However, pS21-EZH2 could still exert its oncogenic function via PRC2-mediated methylation of non-histone proteins such as STAT3, or through associating with AR to function as a transcriptional cofactor, both of which contribute to the context-dependent oncogenic role of pS21-EZH2.
