ABSTRACT
RHO GDP/GTP exchange factors, including VAV1, are considered key protumorigenic factors. Against this paradigm, we have found that VAV1 plays tumor suppressor roles by buffering NOTCH1 signals in thymocytes. The silencing of this pathway contributes to the pathogenesis of T cell acute lymphoblastic leukemia of the early cortical, TLX+ subtype.
KEYWORDS: Rho GTPases, Rho GEFs, Vav1, Notch1, Cbl-b, TLX, lymphoma
The approximately 70 RHO guanosine nucleotide exchange factors (GEFs) that exist in humans are specialized in the catalysis of the transition of RHO GTPases from the inactive (GDP-bound) to the active (GTP-bound) state. The current paradigm in this field holds that RHO GEFs play positive roles in cell transformation. This idea originated from the transforming activity exhibited by most RHO GEFs and, subsequently, by extensive gain- and loss-of-function experiments linking the enzyme activity of these proteins to cancer-related pathobiological programs such as cell cycle regulation, cytoskeletal change, and metastasis.1 This concept became further solidified upon the identification of gain-of-function mutations in some RHO GEF-encoding loci in tumors.2,3 These observations have led to intense efforts to isolate anticancer drugs targeting the catalytic activity of RHO GEFs during the last decade.
The VAV Rho GEF subfamily is composed of three members in humans (VAV1, VAV2, VAV3).4 These proteins exhibit a multidomain structure that allows them to activate both catalysis-dependent and independent pathways (Fig. 1A). For example, in lymphocytes, VAV1 can catalyze Rho GDP/GTP exchange and, at the same time, elicit the calponin homology domain-mediated stimulation of the nuclear factor of activated T cells through a phospholipase Cγ-dependent mechanism (Fig. 1A).4 Analyses of Vav family knockout mice have demonstrated that these proteins play crucial roles in the immune, cardiovascular, and nervous systems.4 In addition, they confirmed the importance of these proteins for the development of a variety of tumors.4-6 Recent data have also unveiled the presence of gain-of-function VAV1 mutations in peripheral T cell leukemia.3 Hence, in agreement with the existing paradigm for the RHO GEF family, it was commonly assumed that VAV proteins were involved in protumorigenic pathways in cancer cells.4
Figure 1.
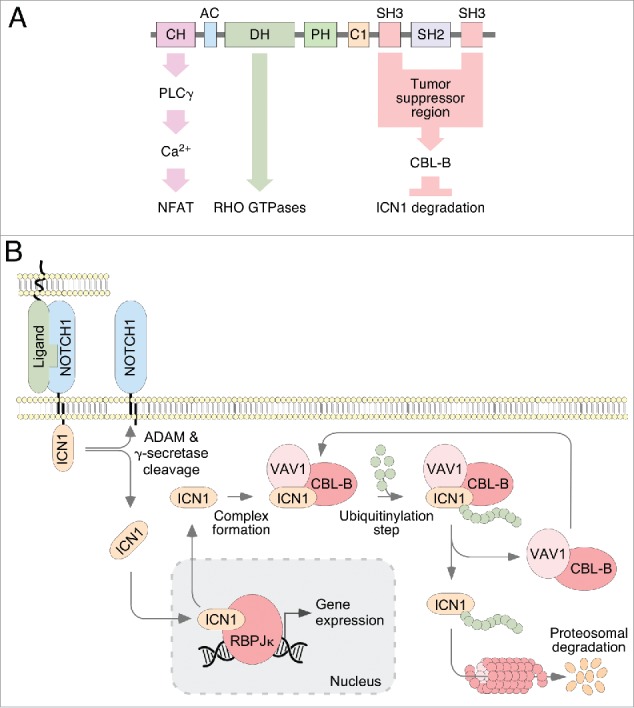
VAV1 signaling in lymphocytes. (A) General structure of VAV proteins and examples of VAV1 catalysis-dependent (green arrow) and independent pathways. CH, calponin homology; AC, acidic region; DH, DBL homology; PH, pleckstrin homology; C1, C1 type zinc finger; SH3; SRC family homology 3; SH2, SRC family homology 2; PLC γ, phospholipase C γ; NFAT, nuclear factor of activated T cells; CBL-B, Cas-Br-murine ecotropic retroviral transforming sequence 2; ICN1, intracellular domain of Notch1. (B) The new VAV1 tumor suppressor pathway involved in the downregulation of ICN1 signaling in immature T cells. ADAM, a disintegrin and metalloproteinase; RBPJk, recombination signal binding protein for immunoglobulin kappa J region. Further details are given in the main text.
Given this functional archetype, it came as a total surprise the recent finding that VAV1 can work as a tumor suppressor in lymphocytes.7 As it usually happens in these cases, the initial clue that led to this discovery was made by serendipity: during experiments aimed at investigating the role of VAV1 in autoimmunity and cancer, we unexpectedly found that Vav1–/– mice developed at high frequency an immature, T cell receptor negative (TCR–) subtype of T cell acute lymphoblastic leukemia (T-ALL) when they aged or, in a more accelerated manner, upon being treated with carcinogens. The mechanistic dissection of this phenotype allowed us to discover a new VAV1-dependent pathway involved in the buffering of NOTCH1 signals in thymocytes (Fig. 1A and B). This was another surprise, given that the RHO GTPase and NOTCH1 pathways are not usually interconnected in cells. It is known that the NOTCH1 route, the most mutated pathway in T-ALL cases,8 is subjected to a very tight regulation by endoproteolytic, bidirectional cytoplasmic-nuclear shuttling, and degradation steps (Fig. 1B). During the activation phase, two proteolytic cleavages of the ligand-bound receptor leads to the release of the intracellular domain of NOTCH1 (ICN1). This fragment then moves to the nucleus and, upon binding to the recombination signal binding protein for the immunoglobulin kappa J region, favors the expression of genes involved in proliferation, metabolism, and lineage commitment. At the end of the stimulation cycle, ICN1 is transferred back to the cytosol, ubiquitinylated, and degraded at the proteosome. Our study revealed that VAV1 participates in this silencing phase by favoring the physical interaction of ICN1 with the E3 ubiquitin ligase CBL-B (Cas-Br-murine ecotropic retroviral transforming sequence 2) in the cytosol. This association, in turn, favors the CBL-B-mediated ubiquitinylation of ICN1 and its subsequent proteosomal degradation (Fig. 1B). This VAV1-dependent pathway is catalysis-independent and only requires the integrity of the two VAV1 SRC homology 3 domains to be engaged in cells (Fig. 1A). In agreement with this regulatory model, the elimination of the Vav1 gene in mice results in increased ICN1 abundance in immature T cells, exacerbated ICN1 signaling and, upon the accumulation of extra genetic lesions, the development of Notch1-dependent T-ALL. Perhaps more importantly, we found that this VAV1-dependent suppressor pathway is actively silenced in human T-cell leukemia homeobox protein-positive (TLX+) T-ALL. This downmodulation is mediated by the direct repression of the VAV1 gene by the transcriptional repressors of the TLX family, the oncogenic drivers responsible for the development of this T-ALL clinical subtype in humans.8 The reexpression of VAV1 results in rapid antiproliferative and apoptogenic effects in all the TLX+ T-ALL cell lines and patient-derived cells tested so far, indicating that the silencing of the VAV1 suppressor pathway is critical for the pathogenesis of this leukemia subtype. Interestingly, the human TLX+ T-ALL and the leukemia developing in Vav1–/– mice share in common the arrest of the transformed T cells in immature, TCR– stages.
Current evidence suggests that other RHO GEFs can also act as tumor suppressors. For example, we have found that Vav3–/– mice develop some type of tumors at higher frequencies than controls. However, this function seems to be different from the VAV1-dependent suppressor pathway reviewed here (M.C. and X.R.B., unpublished). The TIAM1 GEF is another suspect, given its recent implication in the repression of the protumorigenic YAP1–WWTR1 (also known as TAZ) pathway.9 Finally, the recent finding in tumors of recurrent loss-of-function mutations in ARHGEF10 family genes also suggest the potential implication of these GEFs in antitumorigenic pathways.10 Time will tell whether these suspects or other RHO GEFs act as tumor suppressors and, if that were the case, the specific tumor subtypes, structural domains, and signaling mechanisms involved.
Abbreviations
- CBL-B
Cas-Br-murine ecotropic retroviral transforming sequence 2
- GEF
Guanosine nucleotide exchange factor
- ICN1
Intracellular domain of Notch1
- T-ALL
T cell acute lymphoblastic leukemia
- TCR
T cell receptor
- TLX
T-cell leukemia homeobox protein
Funding Statement
Castilla-Leon Government (BIO/SA01/15, CSI049U16), Spanish Ministry of Economy and Competitiveness (MINECO) (SAF2015-64556-R, RD12/0036/0002), Worldwide Cancer Research (14-1248), Ramon Areces Foundation, and Spanish Society against Cancer (GC16173472GARC). Spanish governmental funding is partially supported by the European Regional Development Fund.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors thank M. Dosil and A. Bigas for comments on the manuscript.
References
- 1.Vigil D, Cherfils J, Rossman KL, Der CJ. Ras superfamily GEFs and GAPs: validated and tractable targets for cancer therapy? Nat Rev Cancer. 2010;10:842–57. doi: 10.1038/nrc2960. PMID:21102635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berger MF, Hodis E, Heffernan TP, Deribe YL, Lawrence MS, Protopopov A, Ivanova E, Watson IR, Nickerson E, Ghosh P, et al.. Melanoma genome sequencing reveals frequent PREX2 mutations. Nature. 2012;485:502–6. doi: 10.1038/nature11071. PMID:22622578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abate F, da Silva-Almeida AC, Zairis S, Robles-Valero J, Couronne L, Khiabanian H, Quinn SA, Kim MY, Laginestra MA, Kim C, et al.. Activating mutations and translocations in the guanine exchange factor VAV1 in peripheral T-cell lymphomas. Proc Natl Acad Sci USA. 2017;114:764–9. doi: 10.1073/pnas.1608839114. PMID:28062691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bustelo XR. Vav family exchange factors: an integrated regulatory and functional view. Small GTPases. 2014;5:1–12. doi: 10.4161/21541248.2014.973757. PMID: 25483299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Menacho-Marquez M, Garcia-Escudero R, Ojeda V, Abad A, Delgado P, Costa C, Ruiz S, Alarcón B, Paramio JM, Bustelo XR. The Rho exchange factors Vav2 and Vav3 favor skin tumor initiation and promotion by engaging extracellular signaling loops. PLoS Biol. 2013;11:e1001615. doi: 10.1371/journal.pbio.1001615. PMID:23935450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Citterio C, Menacho-Marquez M, Garcia-Escudero R, Larive RM, Barreiro O, Sanchez-Madrid F, Paramio JM, Bustelo XR. The Rho exchange factors Vav2 and Vav3 control a lung metastasis-specific transcriptional program in breast cancer cells. Sci Sig. 2012;5:ra71. [DOI] [PubMed] [Google Scholar]
- 7.Robles-Valero J, Lorenzo-Martin LF, Menacho-Marquez M, Fernandez-Pisonero I, Abad A, Camos M, Toribio ML, Espinosa L, Bigas A, Bustelo XR. A Paradoxical Tumor-Suppressor Role for the Rac1 Exchange Factor Vav1 in T Cell Acute Lymphoblastic Leukemia. Cancer Cell. 2017;32:608–623.e9. doi: 10.1016/j.ccell.2017.10.004. PMID: 29136506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Vlierberghe P, Ferrando A. The molecular basis of T cell acute lymphoblastic leukemia. J Clin Invest. 2012;122:3398–406. doi: 10.1172/JCI61269. PMID:23023710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diamantopoulou Z, White G, Fadlullah MZH, Dreger M, Pickering K, Maltas J, Ashton G, MacLeod R, Baillie GS, Kouskoff V, et al.. TIAM1 Antagonizes TAZ/YAP Both in the Destruction Complex in the Cytoplasm and in the Nucleus to Inhibit Invasion of Intestinal Epithelial Cells. Cancer Cell. 2017;31:621–634.e6. doi: 10.1016/j.ccell.2017.03.007. PMID:28416184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng J, Demeulemeester J, Wedge DC, Vollan HKM, Pitt JJ, Russnes HG, Pandey BP, Nilsen G, Nord S, Bignell GR, et al.. Pan-cancer analysis of homozygous deletions in primary tumours uncovers rare tumour suppressors. Nat Commun. 2017;8:1221. doi: 10.1038/s41467-017-01355-0. PMID:29089486. [DOI] [PMC free article] [PubMed] [Google Scholar]


