Abstract
We have shown previously that male rats develop a conditioned ejaculatory preference (CEP) for females scented with a neutral odor like almond or lemon that is paired with the male's post-ejaculatory reward state during their first and subsequent early sexual experiences. However, preexposing males to the neutral odor alone prior to its pairing with sexual reward results in latent inhibition. Here, we examined the phenomenon of unconditioned stimulus (US) preexposure, in which male rats were preexposed to the ejaculatory reward state either one or five times with scented (ScF) versus unscented (UnScF) females prior to multiple ejaculatory trials with females in the opposite condition (e.g., ScF preexposure received 10 subsequent ejaculatory trials with UnScF, whereas UnScF preexposure received 10 subsequent ejaculatory trials with ScF). As before, mate and partner preference was evaluated in an open field where each male had access to two females, one ScF and the other UnScF. Males that underwent five trials of preexposure did not display a CEP for either female. Conversely, males preexposed once to a ScF, and later trained with UnScF developed a preference for the latter, whereas males preexposed once to the UnScF, and then trained with ScF did not show a preference for any of the females. Subsequent exposure to the odor cue alone revealed different patterns of brain activation in areas related to sexual behavior that depended on the animal's group membership. Altogether, these findings demonstrate the pivotal role of first sexual experiences in the establishment of future sexual partner preference in the male rat, and suggest an innate preference for estrous odors over neutral odors that can become conditioned subsequently as predictors of sexual reward.
There are many cues that naturally and instinctively drive animals toward conspecifics, especially when attempting to recognize a sexually receptive partner. For example, female rats spend more time among gonadally intact males compared to castrated males (e.g., Gilman and Westbrook 1978). Likewise, male rats are naturally driven toward odors from sexually receptive versus nonreceptive females (e.g., Bakker et al. 1996). These cues appear to be hard-wired and driven by hormonally mediated neural systems within certain hypothalamic and limbic structures (see Pfaus et al. 2003; Pfaus 2009). However, the importance of these cues tends to diminish after baseline rates of sexual responding have been achieved. For example, male rats spend more time in olfactory investigation of sexually receptive females during their first sexual experience with them compared to subsequent experiences (Kagan and Beach 1953; Carr et al. 1962; Stern 1970; Pfaus et al. 2001). Habituation appears to develop in male rats to natural sex odors from familiar females (Carr et al. 1970), although presenting males with novel females can result in the reinitiation of anogenital investigation of the female (Carr et al. 1970; Stern 1970). Similarly, although presenting male rats with a different receptive female during each multiejaculatory test results in a precipitous decline in anogenital investigations prior to the initiation of copulation, subsequent presentation of sexually nonreceptive females results in a vigorous reinitiation of anogenital investigations (Pfaus and Pinel 1989).
Animals are also equipped with associative learning mechanisms that allow them to predict and enact biologically relevant changes in their internal state from environmental cues. These associations are formed by Pavlovian conditioning contingencies (e.g., Rescorla and Wagner 1972) in which neutral cues that become conditioned stimuli (CSs), like neutral odors, acquire associative strength after being paired with biologically relevant cues, or unconditioned stimuli (USs), like the sexual arousal and reward states induced before and after ejaculation, respectively (Zamble et al. 1986; Kippin and Pfaus 2001a; Tenk et al. 2009). For instance, male Japanese quails associated a CS more readily when they had access to copulate with a receptive female than when they were only exposed to it (Holloway and Domjan 1993). Furthermore, male rats have been shown to develop a conditioned ejaculatory preference (CEP) toward females bearing a neutral odor like almond or lemon that has been associated previously with the post-ejaculatory reward state (e.g., Kippin et al. 1998; Kippin and Pfaus 2001a) However, given that the procedure to develop a CEP requires several training trials, the changes that could possibly occur during first sexual experiences and their effects on a subsequent trained CEP are buried in the subsequent trials. Consequently, the impact of first sexual experiences on the development of CEP remains largely unexplored, although blocking opioid transmission by systemic treatment with the opioid receptor antagonist naloxone during training can block the formation of both CEP (Ismail et al. 2009) and sexually conditioned place preference (Ågmo and Berenfeld 1990; Mehrara and Baum 1990).
The impact of the first exposure to a US on the subsequent ability to associate a neutral stimulus with the US, phenomenon known as US preexposure, results in a great retardation or outright blocking of the establishment of a CR if a CS is paired with the US in a context in which the animal was previously exposed to the US alone (e.g., Randich and LoLordo 1979). Significant US preexposure effects have been demonstrated for conditioned taste aversions (e.g., Clasen et al. 2017) and conditioned fear (e.g., Frankland et al. 2004), but not learned immunosuppression (Lueckemann et al. 2016). As an early example, Taylor (1956) conditioned the blinking response of human participants using an air puff signaled by a light. Before training, one group received several presentations of the air puff in three different intensities to the cornea of their eyes without the light. The number of eye-blink responses was greater in the group that was not preexposed to the air puff, whereas in the preexposed group the number of eye-blink responses was in an indirect correlation with the intensity of the air puff during the preexposure phase. Two hypotheses have been offered to explain this phenomenon, one associative and the other nonassociative. The former proposes that the US preexposure effect is due primarily to an association between the US and cues in the context in which the initial US preexposure takes place prior to training. The contextual cues trained with the initial exposure to the US blocks the subsequent association of other potential CSs trained in that context (e.g., Rescorla and Wagner 1972; Randich and LoLordo 1979). The latter claims that by preexposing the US, there is a reduction in the initial emotional reactivity of the animal's response due to general habituation that reduces the salience of the US, and thereby attenuates subsequent excitatory conditioning (e.g., Rankin et al. 2009). In terms of the sexual reward state induced by ejaculation in male rats, the associative explanation predicts that contextual cues associated with the first experience(s) of copulation and ejaculation (or other rewarding aspects of sexual interaction with a female) would come to block subsequent attempts to associate a discrete cue, such as a neutral odor, with the ejaculatory reward state. Alternatively, the nonassociative hypothesis would predict that the emotional quality of repeated ejaculations in the same context decreases after the first several experiences, thus driving down the associative strength conferred from the US to the CS as a predictor.
Previously, we assessed if preexposure to the neutral odor (almond) used as the CS in the development of CEP could alter conditioning via latent inhibition (Quintana et al. 2018). Males were preexposed to a neutral odor (almond) either one or five times before they were trained to develop a CEP based on that odor cue. As in our previous studies, mate and partner preferences were evaluated through an open-field test, where males had access to two sexually receptive females, one scented and another unscented. Males that had been given five trials with the odor alone and subsequently trained to develop CEP based on the odor did not show a preference for either female, whereas males exposed only one time showed a preference for the scented female. After two reconditioning trials were given after the open-field test, the odor alone was presented to the males in the different groups and its ability to activate cellular Fos protein (a marker of neuronal activation) in different brain regions was examined. A significantly greater activation of Fos in the group given one CS preexposure trial in the medial preoptic area (mPOA), ventral tegmental area (VTA), and nucleus accumbens core (NAc Core) relative to the group given five CS preexposure trials was found. In contrast, the group with one CS preexposure had smaller numbers of Fos-positive cells compared to a paired control group in the basolateral amygdala (BLA) and NAc Core and NAc shell (NAc Shell), all of which are a subset of the general pathway that underlies olfactory partner preference conditioning in both male and female rats (Pfaus et al. 2010).
The present study sought to evaluate whether US preexposure would block the subsequent conditioning of CEP in male rats. Sexually naïve male rats were given either one or five sexual experiences to one ejaculation each with either sexually receptive females scented with a neutral almond odor (ScF) or left unscented (UnScF). Subsequently, males were given another 10 tests of sexual behavior with ScF if they were preexposed to an UnScF, or vice versa, after which CEP and other sexual partner preferences were assessed in a large open field with two receptive females, one ScF and one UnScF. As in our previous study, following two reconditioning trials each male was exposed to the almond odor alone for an hour to assess the ability of the CS to activate Fos.
Materials and Methods
Subjects
Males
Ninety-two Long-Evans rats were sexually naïve and weighing ∼250 g at the beginning of the experiment. They were housed in groups of four and two in Plexiglas cages with ad lib access to water and food (Purina Rat Chow). Males were obtained from Charles River Canada (St-Constant, QC, Canada) and kept in a 12 h. reversed light–dark cycle in a room at 21°C.
Females
One-hundred and twenty Long-Evans rats sexually naïve and weighing ∼200 g at the beginning of the experiment were obtained from the same distributor and housed in pairs in the same conditions as males. Females were ovariectomized via bilateral lumbar incisions under ketamine (50 mg/mL)/xylazine (4 mg/mL) anesthesia, mixed at a ratio of 4:3, respectively, approximately 2 wk before the beginning of the experiment. Sexual receptivity was induced by subcutaneous injections of 10 µg estradiol benzoate (Steraloids, injected sc in 0.1 mL of sesame oil) 48 h prior each training session, and 500 µg of progesterone (Steraloids, injected sc in 0.1 mL of sesame oil) 4 h prior to each training session. Stimulus females were scented with 0.6 mL of pure almond extract (Blue Ribbon), split equally in the back of their neck and anogenital region. Different females were assigned to each male randomly for every training session.
Apparatus
All conditioning sessions were conducted in Plexiglas unilevel pacing chambers (38 × 60 × 38 cm) with bedded floors and bisected by a transparent Plexiglas divider with one-hole large enough for the female to cross but not the male, as it has been previously found that pacing copulation where males have restricted access to a family facilitates the development of a CEP (Ismail et al. 2009). The cage bedding was not changed between conditioning sessions, and animals trained with ScF were trained in separate cages and rooms from the ones trained with UnScF. The final copulatory preference test took place in a large open field (123 × 123 × 46 cm) filled with clean bedding. All sessions were recorded and subsequently scored with using a behavioral scoring program (Cabilio 1996) that counted frequencies and latencies of individual sexual behaviors (e.g., mounts, intromissions, and ejaculations; as in Sachs and Barfield 1976; Pfaus et al. 1990; Meisel and Sachs 1995).
Procedure
The common procedure of the experiment is depicted in Figure 1.
Figure 1.
General experimental procedure.
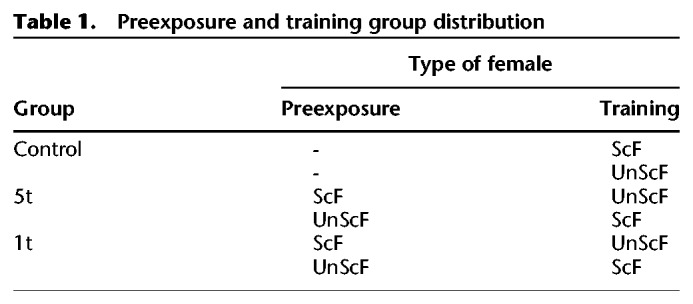
Groups
Males were assigned and equally divided into one of three main groups (see Table 1): five trials of US preexposure (5t), one trial of preexposure (1t), or control groups. During preexposure phase, males in the 5t group copulated five times with receptive females, whereas males in the 1t copulated only once. For this part of the experiment, half of the males in the 1t and 5t copulated with ScF, whereas the other half of males in 5t and 1t copulated with UnScF. Subsequently, during the training trials, males copulated with the opposite female assigned during the preexposure phase. Males in the control group were trained with either ScF or UnScF, only.
Table 1.
Preexposure and training group distribution
Context preexposure
All animals were exposed five times to the chamber in a 4-d interval for ∼30 min prior to the preexposure phase (or prior to conditioning in the case of the control groups), in order to habituate them to the training environment, as it has been shown that a novel environment disrupts copulation in sexually naïve rats (Pfaus and Wilkins 1995).
US preexposure
Preexposure trials consisted of each male copulating in an unilevel pacing chamber with one-hole divider with a receptive female scented or unscented (depending their group), until the first intromission after the first ejaculation.
Conditioning
Following the preexposure phase, all animals were trained to develop a CEP for a sexually receptive female with or without bearing an almond odor (depending on the group), using a similar protocol to the one described in Kippin and Pfaus (2001b). Rats were trained with ScF or UnScF for 10 conditioning sessions at 4-d intervals during the middle third of the dark phase of the light–dark cycle.
Depending on which type of female the males were preexposed to, half of the animals of each of those groups were given 10 subsequent trials with the other type of female (ScF for the males preexposed to UnScF, or UnScF for the males preexposed with ScF; see Table 1).
During each conditioning trial males were placed into the unilevel pacing chamber for 5 min, after which a sexually receptive female was placed into the chamber to copulate freely with the male. Trials were terminated when the male mounted or intromitted with the female after the refractory period that followed the first ejaculation.
Copulatory preference test
Four days after the last conditioning trial, each male was placed in a large open-field (123 × 123 × 46 cm) covered with Beta Chip bedding and allowed to explore for 5 min. Subsequently, two females (ScF and UnScF, randomly assigned) were placed into the open field simultaneously, both equally distant from the male, and were allowed to copulate for 30 min. The test was video recorded and scored subsequently for the choice of female for mounts, intromissions, and ejaculations.
Perfusion
Following the preference test, males were given two reconditioning trials, after which they were exposed for 45 min to 1 mL of the almond odor alone on a gauze pad on the other side of the pacing chamber with the divider. Subsequently, males were injected with euthanyl (120 mg/kg, i.p.) and perfused intracardially with 250 mL of phosphate buffered saline followed by 250 mL of 4% paraformaldehyde. Brains were extracted and post-fixed in 4% paraformaldehyde for 4 h, to be later on stored for 36 h in a 30% sucrose solution. Finally, the brains were frozen, covered in aluminum foil and stored at −80°C.
Fos immunohistochemistry
This analysis was performed as in previous studies (e.g., Kippin et al. 2003). Coronal brain sections were incubated sequentially with 30% w/w hydrogen peroxide (H2O2) in Tris-buffered saline (TBS) for 30 min at room temperature, 3% normal goat serum (NGS) in 0.05% Triton TBS for 90 min at 4°C, rabbit polyclonal anti-Fos (Oncogene Science; diluted 1:75,000) in 0.05% Triton TBS with 3% NGS for 72 h at 4°C, biotinylated goat anti-rabbit IgG (Vector Laboratories 1:200) in 0.05% Triton TBS with 3% NGS for 1 h at 4°C, and avidin-biotinylated–peroxidase complex (Vectastain Elite ABC Kit, Vector Laboratories; diluted 1:55) for 2 h at 4°C. Sections were washed in TBS (35 min) between each incubation. Immunoreactions were stained by sequential treatments at room temperature with 50-mM Tris for 10 min, 3,3′-diaminobenzidine (DAB) in 50-mM Tris (0.1 mL of DAB/Tris buffer, pH 7.8) for 10 min, DAB/3% H2O2 in 50-mM Tris for 10 min, and 8% nickel chloride (400 µL per 100 mL of DAB/Tris buffer H2O2). Sections were mounted on gel-coated slides and allowed to dry, then dehydrated, cleared in Hemo-D, coverslipped and examined under a microscope. Brain sections were examined at 40×, and the number of Fos-positive cells was counted bilaterally from each region from five different sections per rat (Leitz Microscope) using a computerized image-analysis system (ImageJ).
Brain regions related to sexual behavior and CEP were observed for Fos-IR to evaluate the neural activation evoked by the odor cue used during training. The regions examined were similar to those in Kippin et al. (2003), and were defined using the borders in Paxinos and Watson (1998) including the \mPOA (−0.35 mm from bregma), nucleus accumbens shell and core (NAc Shell and NAc Core, respectively, 1.65 mm from bregma), VTA (−6.04 mm from bregma), central nucleus of the amygdala (CeA, −2.80 mm from bregma) and the basolateral nucleus of the amygdala (BLA, −2.80 mm from bregma). An average of Fos-positive cells was calculated from three different slides from each rat (five subjects in each group), for each brain area.
Statistical analyses
A series of mixed design, between-within repeated-measures ANOVAs were conducted separately for each copulatory measure (mounts, intromissions, ejaculation, and latency to the first ejaculation), displayed among males in the four training groups (1t, 5t, Control ScF, and Control UnScF) with the two receptive females (ScF or UnScF) on the final open-field test. For each significant ANOVA, post hoc compassions of the means were made using the Tukey HSD correction to ensure to ensure maximal statistical power while correcting for family-wise error. Furthermore, partial eta square () was calculated as effect size for each comparison. Additionally, a 1 × 2 chi square (χ2) analysis was conducted for the percentage of first ejaculation choice for each group, and a 2 × 2 χ2 analysis to contrast the ejaculatory preference between the control groups. Furthermore, Cramer's V and Phi (φ) effect sizes were conducted as effect size for the 1 × 2 and 2 × 2 χ2 analyses, respectively.
For the Fos-IR results, the mean of Fos-IR-positive cells for each brain area was compared separately among the experimental groups using independent samples t-test with a Bonferroni correction of the alpha level to control for the family-wise error (Miller 1966). Since four comparisons were conducted within each brain area, the α level was set at 0.0125 (0.05/4 = 0.0125) for statistically significant differences. Only in the comparisons between the control groups, the α level was kept at 0.05, since those independent t tests compared only two groups. Cohen's d effect size statistics were also calculated as a measure of effect size. The following comparisons between groups were conducted for each brain area of interest: 5t ScF versus 1t ScF, 5t UnScF versus 1t UnScF, 5t ScF versus 5t UnScF, 1t ScF versus 1t UnScF, and ScF control versus UnScF control. The latter comparisons were run separately.
Results
Behavioral analysis
Four males were not included in the final statistical analyses since they did not copulate in the open-field test. Although five min of exploration in the open field have previously been used effectively as a period of acclimation for males before the open-field test (e.g., Kippin and Pfaus 2001b), it is believed that natural differences in novelty aversion vary in such ways that some animals are affected more than others, and likely show fear responses (such as hugging the walls of an open field) as we observed.
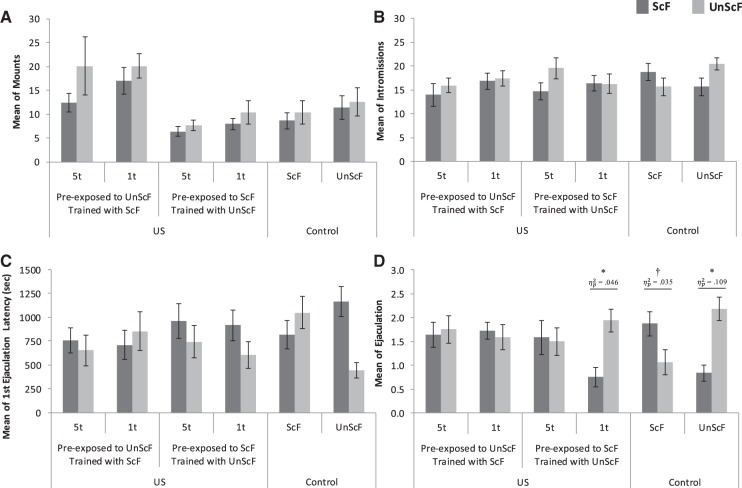
The scores for the different copulatory behavior by female for all groups during the open-field test are shown in Figure 2. Males did not display consistent differences for the distribution of mounts or intromissions between the females among groups. The reliability of these observations was corroborated by two independent 6 (Group: 1t UnScF, 5t UnScF, 1t ScF, 5t ScF, Control ScF, and Control UnScF) × 2(Female: ScF, UnScF) repeated-measure ANOVA. No statistically significant interaction between Female × Group was found for mounts, F(5,86) = 0.609, P = 0.693, ; nor for intromissions, F(5,86) = 1.524, P = 0.191, .
Figure 2.
Mean of copulatory behaviors (±SEM) per group during the open-field test. (†) P < 0.01; (*) P < 0.05; = partial eta square.
As shown on panels C and D of Figure 2, the control and the 1t trained with UnScF groups appeared to take less time to ejaculate first with the female they were trained with and chose to ejaculate more with her. The reliability of these observations was partially confirmed by two independent 6 (Group: UnScF 1t, UnScF 5t, ScF 1t, ScF 5t, Control ScF, and Control UnScF) × 2(Female: ScF, UnScF) repeated-measure ANOVAs. On one hand, no statistically significant interaction between Female × Group was found for the latency of first ejaculation mean, F(5,86) = 1.796, P = 0.122, . However, a significant interaction between Female × Group was found for the mean of ejaculations, F(5,86) = 3.173, P = 0.011, . Post hoc comparisons using the Tukey HSD correction revealed that the control UnScF group displayed a statistically significantly higher mean ejaculation toward the UnScF (M = 2.19) than to the ScF (M = 0.688, P < 0.05, ), whereas the ScF control displayed a statistically marginally higher mean of ejaculations toward the ScF (M = 1.63) than to the UnScF (M = 1.06, P < 0.1, ). Also, the 1t group preexposed to ScF and later on trained with UnScF displayed a statistically significantly higher mean ejaculation toward the UnScF (M = 1.94) than the ScF (M = 1.0, P < 0.05, ).
The percentage of males that chose ScF or UnScF for their first ejaculation is shown in Figure 3. Males in both 5t groups and in the 1t UnScF group and later on trained with ScF, did not show a significant choice for first ejaculation for either of the females. However, males in ScF 1t group and later on trained with UnScF and both control groups chose to ejaculate first with the female they were training with. These observations were partially confirmed by χ2 analyses. No significant differences were found in percentage of first ejaculation choice for the UnScF 5t group trained with ScF group, χ2(1) = 0.25, P = 0.617, V = 0.125; nor for the ScF 5t group trained with UnScF, χ2(1) = 0.25, P = 0.617, V = 0.125; neither for the UnScF 1t trained with ScF, χ2(1) = 0, P > 0.9, V = 0. However, statistically significant differences were found in the control UnScF group, χ2(1) = 4.0, P = 0.046, V = 0.5; yet no significant differences were found for the ScF 1t trained with UnScF group, χ2(1) = 2.25, P = 0.134, V = 0.375; nor on the Control ScF, χ2(1) = 1, P = 0.317, V = 0.25. However, the effect sizes demonstrated a clear trend for the latter two. Furthermore, a 2 × 2 χ² analysis between the two control groups revealed that, overall, they statistically significantly preferred different females to ejaculate first with, χ2(1) = 4.571, P = 0.033, φ = 0.315.
Figure 3.
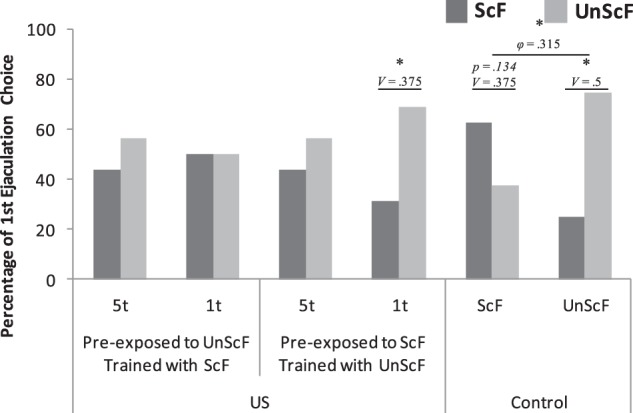
Percentage of first ejaculation choice per group during the open-field test. (*) P < 0.05; V = Cramer's V; φ = phi.
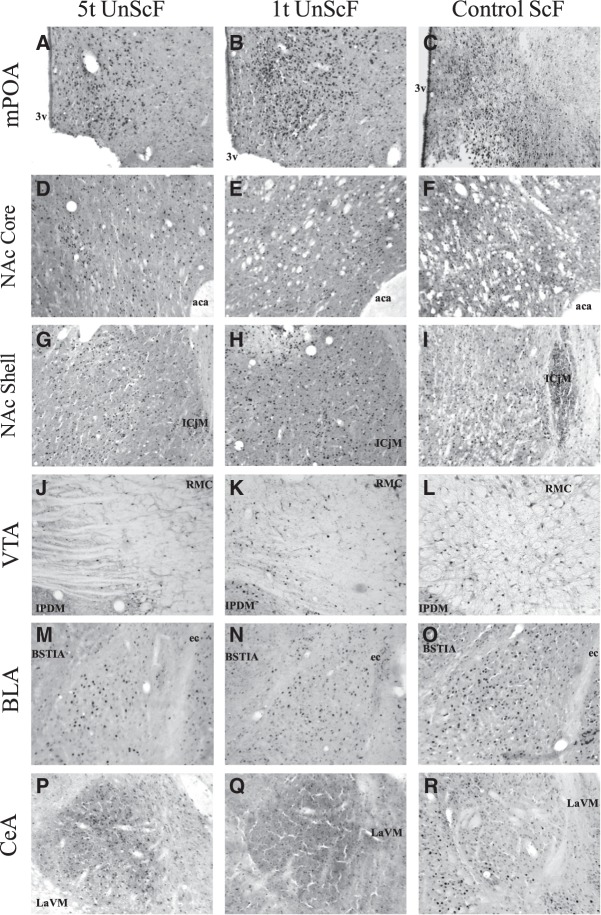
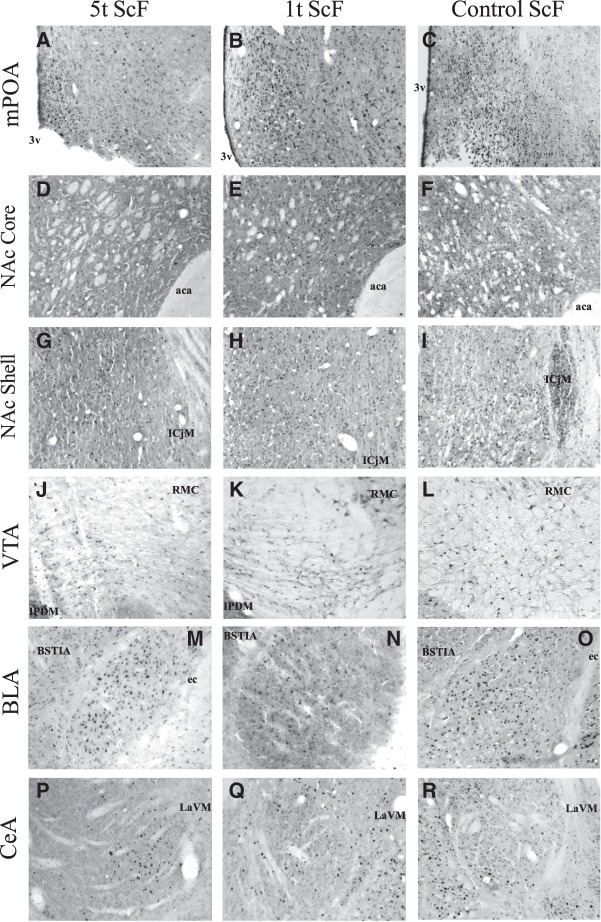
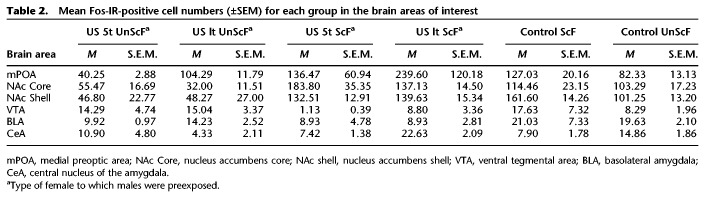
Fos-IR
Figures 4 and 5 show an example of the Fos-IR in each of the brain area of interest for each of the groups. Table 2 displays the mean Fos-IR-positive cell numbers (±SEM) for each group in the brain areas of interest. As shown in Figure 4, following exposure to the odor cue in the group of males preexposed to UnScF and later on trained with ScF, both 5t and 1t groups had a lower Fos-IR activation than the ScF control group in the NAc Core and Shell, VTA, and BLA; whereas only the latter had a higher Fos-IR activation in the mPOA than the ScF control group. As shown in Figure 5, following exposure to the odor cue in the group of males preexposed to ScF and later on trained with UnScF, the 1t group had a higher Fos-IR activation than the 5t group in the mPOA, BLA, and CeA, whereas only in the CeA did the 1t group have a higher Fos-IR activation than the ScF control group. The reliability of these observations was partially confirmed by t-test with a Bonferroni correction and d effect sizes.
Figure 4.
Fos immunoreactivity (Fos-IR) following exposure to the sexually conditioned odor before perfusion in males preexposed five times to a unscented female before being trained with scented females (5t UnScF), males preexposed one time to an unscented female before being trained with scented females (1t UnScF), and control males trained with scented females (CC+), in brain areas of interest. Pictures were taken accordingly to Paxinos and Watson (1998) coordinates in the mPOA: ±−0.40 mm from Bregma (A–C); Nucleus accumbens Core and Shell (NAc Core/Shell): ±1.70 mm from Bregma (core: D–F, shell: G–I); VTA: ±−6.04 mm from Bregma (J–L); the BLA: ±−3.14 mm from Bregma (M–O), and in the Central nucleus of the amygdala (CeA): ±−2.80 mm from Bregma (P–R). Abbreviations used in the figure: (3v) third ventricle, (aca) anterior commissure, (ICjM) major islands of Calleja, (RMC) magnocellular part of red nucleus, (IPDM) dorsomedial interpeduncular nucleus, (ec) external capsule, (BSTIA) intra-amigdaloid division of the bed nucleus of the stria terminalis, (LaVM) ventromedial part of the lateral amygdaloid nucleus. See Table 2 for M ± S.E.M.
Figure 5.
Fos immunoreactivity (Fos-IR) following exposure to the sexually conditioned odor before perfusion in males preexposed five times to a scented females before being trained with unscented females (5t ScF), males preexposed one time to a scented female before being trained with unscented females (1t ScF), and control males trained with scented females (CC+), in brain areas of interest. Pictures were taken accordingly to Paxinos and Watson (1998) coordinates in the mPOA: ±−0.40 mm from Bregma (A–C); Nucleus accumbens Core and Shell (NAc Core/Shell): ±1.70 mm from Bregma (core: D–F, shell: G–I); VTA: ±−6.04 mm from Bregma (J–L); the BLA: ±−3.14 mm from Bregma (M–O), and in the Central nucleus of the amygdala (CeA): ±−2.80 mm from Bregma (P–R). (3v) third ventricle, (aca) anterior commissure, (ICjM) major islands of Calleja, (RMC) magnocellular part of red nucleus, (IPDM) dorsomedial interpeduncular nucleus, (ec) external capsule, (BSTIA) intraamigdaloid division of the bed nucleus of the stria terminalis, (LaVM) ventromedial part of the lateral amygdaloid nucleus. See Table 2 for M ± S.E.M.
Table 2.
Mean Fos-IR-positive cell numbers (±SEM) for each group in the brain areas of interest
mPOA
Males in the 5t ScF group had a statistically significantly lower Fos-IR than the 1t ScF group, t(8) = −5.28, P < 0.01, d = 7.462, whereas no statistically significant differences were found between males from the 5t UnScF compared to males in the 1t UnScF group, t(8) = 0.765, P > 0.05, d = 1.082. No statistically significant differences were found comparing males in the 5t ScF versus 5t UnScF, although the effect size revealed a difference of a high magnitude, t(8) = −1.577, P > 0.05, d = 2.23; nor for males from the 1t ScF versus 1t UnScF groups, t(8) = −1.12, P > 0.05, d = 1.585.
VTA
No statistically significant differences were found between males in the 5t UnScF group compared to males in the 1t UnScF group, t(8) = −0.13, P > 0.05, d = 0.182; nor did males in the 5t ScF compared to males in the 1t ScF group, although the effect size revealed a difference of a high magnitude, t(8) = −2.26, P = 0.053, d = 3.207. Males in the 5t UnScF group had a statistically marginally higher Fos-IR than the 5t ScF group, t(8) = −2.76, P = 0.024, d = 3.913; whereas no statistically significant differences were found comparing males from the 1t ScF versus 1t UnScF groups, t(8) = 1.31, P > 0.05, d = 1.519.
NAc shell
No statistically significant differences were found comparing males in the 5t ScF versus 1t ScF, t(8) = 1.22, P > 0.05, d = 0.502; nor for males from the 5t UnScF versus 1t UnScF groups, t(8) = 1.55, P > 0.05, d = 0.059. Males in the 5t ScF group had a statistically significantly higher Fos-IR than the 5t UnScF group, t(8) = 3.27, P < 0.01, d = 4.631; just like males in the 1t ScF group had a statistically significantly higher Fos-IR than the 1t UnScF group, t(8) = 5.67, P < 0.001, d = 4.161.
NAc core
No statistically significant differences were found comparing males in the 5t ScF versus 1t ScF, t(8) = −0.35, P > 0.05, d = 1.727; nor for males from the 5t UnScF versus 1t UnScF groups, t(8) = 0.04, P > 0.05, d = 1.637. Males in the 5t ScF group had a statistically significantly higher Fos-IR than the 5t UnScF group, t(8) = 3.28, P < 0.01, d = 4.643; whereas males in the 1t ScF group had a statistically significantly higher Fos-IR than the 1t UnScF group, t(8) = 2.94, P = 0.0185, d = 8.031.
BLA
No statistically significant differences were found comparing males in the 5t ScF versus 1t ScF, t(8) = −1.6, P > 0.05, d = 0; males from the 5t UnScF versus 1t UnScF groups, t(8) = −0.01, P > 0.05, d = 2.257; males in the 5t ScF versus 5t UnScF, t(8) = 0.2, P > 0.05, d = 0.287; nor between males from the 1t ScF versus 1t UnScF groups, t(8) = −1.4, P > 0.05, d = 1.986.
CeA
Males in the 5t ScF group had a statistically significantly lower Fos-IR than the 1t ScF group, t(8) = −6.07, P < 0.001, d = 8.589; whereas no statistically significant differences were found comparing males from the 5t UnScF versus 1t UnScF groups, t(8) = 1.25, P > 0.05, d = 1.772. No statistically significant differences were found comparing males in the 5t ScF versus 5t UnScF, t(8) = −0.69, P > 0.05, d = 0.985; whereas males in the 1t ScF group had a statistically significantly higher Fos-IR than the 1t UnScF group, t(8) = 6.15, P < 0.001, d = 8.714.
As shown in Table 2, following exposure to the odor cue, males in the ScF control group had a higher mean of Fos-IR than the UnScF control group in all brain areas, except in the CeA. The reliability of these observations was partially confirmed by an independent t-test and d effect sizes.
In the mPOA, males in the ScF control group had a statistically marginally higher Fos-IR than the UnScF group, although the effect size revealed a difference of a high magnitude, t(8) = 1.99, P = 0.08, d = 1.18. In the NAc Shell, males in the ScF control group had a statistically significantly higher Fos-IR than the UnScF group, t(8) = 3.11, P < 0.01, d = 4.392. In the CeA, males in the ScF control group had a statistically significantly lower Fos-IR than the UnScF group, t(8) = −4.56, P < 0.001, d = 3.823. No statistically significant differences were found in any other area, Ps < 0.05.
Discussion
The present study evaluated the impact of US preexposure on the development of CEP in the male rat. Males that were given five sexual experiences with females prior to training (with or without the odor) did not develop a CEP for the subsequently familiar female. Furthermore, the same disruption was found even when only one trial of preexposure was given, but only when the female was not bearing an odor. If the female was scented then males developed a CEP for the female they were trained with. The Fos-IR analyses demonstrated a differential pattern of neural activation regarding the amount of preexposure and the type of female with whom the males underwent the training phase. When compared to the ScF control group, these patterns argue for a differential role on the CS–US associability depending on when these are paired. Previous studies have found that CEP develops when male rats have repeated multi-ejaculatory trials with sexually receptive females bearing a neutral odor such as almond or lemon (e.g., Kippin et al. 2001; Kippin and Pfaus 2001a,b). This effect can be impaired or inhibited when the odor cue is preexposed five times, but not one time, before conditioning (Quintana et al. 2018). Together these studies show that the development of CEP follows Pavlovian rules regarding latent inhibition and US preexposure (e.g., Lubow and Moore 1959; Randich and LoLordo 1979, respectively).
Behavioral analyses
One of the main findings of the present study was the disruption of the CEP toward the familiar female in the 5t groups preexposed to the US. As previously mentioned, there are two perspectives to interpret the behavioral results on US preexposure, the associative and the nonassociative. Moreover, Mis and Moore (1973) concluded that the decremental effect of preexposing animals to a US was in direct correlation to the number of US presentations as well as the US intensity, and inversely correlated with the interval between the last preexposure trial and the first conditioning trial. Either through blocking or the reduction of the initial emotional reaction toward the US, these findings showed that preexposing sex five times before training a CEP is enough to disrupt it corroborating similar findings on the effect of preexposure of cues related to the training conditions before the conditioning for CEP (Quintana et al. 2018). This was expected given that it has been shown previously that five multiejaculatory trials of 30 min are the minimal amount of conditioning experience necessary to establish a CEP based on neutral odor cue in male rats (Kippin and Pfaus 2001b). Thus, if five trials are enough to establish a CEP, they are also enough to establish an association with the context that could further impair other association trained in the same context. Furthermore, the present study also found a lack of CEP in males preexposure one time to an UnScF and later trained with ScF. It could be possible that the first experience with sexual reward involving ejaculation is strong enough, and an inter trial interval of 4 d is short enough to establish a Context-US connection that will hinder the subsequent association between any CS–US presentations in which a CEP can be based. However, no available data establish how long lasting these associations may be, nor do they show for how long the disrupting effect of US or CS preexposure might last. Nevertheless, the preexposure effect on the CEP findings, particularly the disruption of the CEP in the 1t group preexposed to an UnScF, confirm the powerful impact of first sexual experiences on the ability to associate a CS with sexual reward.
Considering one copulatory preexposure to the ScF did not block subsequent conditioning to the UnScF, it may be surprising that 1t preexposure to the UnScF blocked later training with ScFs. This may be an example of belongingness or preparedness, a phenomenon where certain CS–US associations are easier to be established than others (Garcia and Koelling 1966; Seligman 1970). This phenomenon was first described in the taste aversion literature, where facilitated acquisition and resistance to extinction was observed between taste and gastrointestinal distress. Likewise, natural pheromonal cues from sexually receptive females are innately preferred by male rats (Carr et al. 1965; Bressler and Baum 1996), eliciting general and sexual arousal (Sachs 1997) and increasing testosterone and luteinizing hormones levels in plasma (Graham and Desjardins 1980). Therefore, it appears that rats may have evolved to display specific “prepared” association between estrous odors and sexual partner receptivity (see Cook et al. 1986). The absence of a disruption in CEP in the males given 1 preexposure to the ScF and trained subsequently with UnScF suggests that a neutral odor requires enough conditioning in order to reach critical salience, unlike prepared cues like estrous odors (also see Kippin et al. 2001) where males did not develop a preference for a ScF after only one training trial. Indeed, estrous odors are able to activate mesolimbic dopamine release unconditionally in the NAc, whereas neutral odors must be paired repeatedly with the post-ejaculatory reward state to induced dopamine release (Pfaus et al. 2012).
Fos-IR
Analysis of Fos-IR in the brain areas of interest showed a differential pattern of activation depending on the amount of preexposure and training conditions.
As depicted on Figure 4, following exposure to the odor cue, on one hand, males in the 5t group preexposed to UnScF and later on trained with ScF had a lower mean of Fos-positive cells in the mPOA and CeA than the 1t group preexposed and trained under the same conditions. Additionally, males in the 5t group had a lower mean of Fos-positive cells in the mPOA compared to the ScF control group, whereas males in the 1t group had a higher mean of Fos-positive cells in the CeA compared to the ScF control group. On the other hand, as depicted in Figure 5, males in the 5t group preexposed to ScF and later on trained with UnScF did not significantly differ from the 1t group preexposed and trained in the same conditions in their mean of Fos-positive cells in any of the brain areas of interest, expect for the VTA, where the former had a lower mean of Fos-positive cells than the latter. Furthermore, both of them had a lower mean number of Fos-positive cells than the ScF control group in the NAc Core and Shell, and only did the 5t group have a lower mean of Fos-positive cells in the VTA compared to the ScF control group.
Previously, Kippin et al. (2003) examined the Fos-IR in males trained to associate an olfactory cue with sexually receptive females, and compared this to the activation elicited by estrous odors. Following exposure to the estrous odors there was an increase in Fos-IR in the accessory olfactory bulb, medial amygdala, medial bed nucleus of the stria terminalis, mPOA, ventromedial hypothalamus, VTA, and both NAc core and shell. Following exposure to the sexually conditioned odor, Fos-IR increased in the piriform cortex, BLA, NAc core, and the anterior portion of the lateral hypothalamic area. The common activation of the NAc core lead to the authors to suggest that estrous and sexually conditioned odors are processed by a common neuronal system in that brain area (Kippin et al. 2003). In the present study, US preexposure of either, ScF or UnScF, led to a lower Fos-IR compared to the control ScF group in almost all brain areas studied. It is worth mentioning that Kippin et al. (2003) used bilevel chambers, where the chasing dynamic between male and female is completely different from that in the unilevel pacing chambers used in this study (Ismail et al. 2009).
A companion study of CS cue preexposure prior to CEP training yielded similar decrements in Fos-IR in several brain areas (Quintana et al. 2018). Therefore, a general decrement in Fos-positive cells elicited by the odor in comparison to the ScF control group in the brain areas of interest may be due to a different pattern of associability for the odor, depending the contingency between the preexposure and training phase. It is interesting that the mPOA and VTA/NAc appear to be processing the odor differently. First, the mPOA seems sensitive to the odor in a combined function between the number of trials of preexposure and when this was paired with sexual reward, whereas the VTA and NAc seem to be processing the odor mostly according to the training contingencies. This pattern suggests that the mPOA may be balancing the reward prediction value, whereas the VTA and NAc predict the contingencies of when the odor may be predicting the reward. Furthermore, the CeA appears to be doing the opposite than the ScF control, also doing the opposite than what the mPOA may be doing.
The pattern by which this general decrement varies depending on the contingencies of preexposure and training are discussed by brain area.
mPOA
The mPOA is a critical brain region that controls male sexual arousal and behavior, where every sensory modality sends indirect inputs (see Dominguez and Hull 2005; Hull and Rodriguez-Manzo 2009). More specifically, it is believed that the mPOA controls erection and copulatory behavior, but not purely motivational aspects of sexual behavior (Everitt 1990). Lesions have shown to impair or completely abolish male sexual behaviors (Hull et al. 2006), and electrophysiological stimulation has shown to facilitate it, yet not reverse sexual satiation (Rodriguez-Manzo et al. 2000). More specifically, Fos-IR has shown to increase in the mPOA in response to copulatory stimulation (Baum and Everitt 1992), and although it has not shown to increase due to the exposition of a neutral cue paired with sexual reward in male rats (Kippin et al. 2003), the ScF control group of this study had a higher Fos-IR than the UnScF control group. Thus, a lower Fos-IR in males preexposed five times to ScF and later on trained with UnScF than the ones preexposed one time may suggest that the odor may have been impaired from fostering rewarding associations with copulation given the preexposure manipulation, thus impairing a CEP otherwise found in the UnScF control group. This highlights the amount of training as a factor that influences trained associations as seen before in the parametric parameters of the development of a CEP (Kippin et al. 2001). Furthermore, no decrement of Fos-IR was found in the mPOA in males preexposed to UnScF. Interestingly, males preexposed 1t and later on trained with ScF had a higher activation than all groups. This suggests that in the mPOA, the pattern of associability for a neutral olfactory cue depends on the contingencies of training, just like the amount of training. However, other prepotent cues like estrous odors would be more readily or easily be associated with the rewarding aspects of sex, as demonstrated by disruption in the CEP and high Fos-IR found in the mPOA of males preexposed 1t and later on trained with ScF.
NAc core and shell
As one of the terminal brain region of the mesolimbic dopaminergic pathway, the NAc has been associated with reinforcement and appetitive behavior, and attention to sexual incentive cues (e.g., Hull et al. 2006). As a CS–US integrator, the mesolimbic terminal regions focus the necessary attention on conditioned incentive cues to direct motor output toward them enabling animals to engage in copulation, which ultimately results in ejaculation. This allows the sexual reward state to be cued and predicted by a CS (e.g., Pfaus et al. 2010). Lesions of this area increased the refractory period and decreased noncontact erection, yet not impeding males from copulating (Liu et al. 1998). Moreover, using excitotoxic lesions in the NAc of male rats, several behaviors like mounts, intromissions, noncontact erections, among others, were partially hindered, yet not abolished (Kippin et al. 2004). Recordings from the NAc of male rats after being exposed to novel female estrous odors showed a greater response than the estrous of a familiar female (Wood et al. 2004). Using microdialysis, another study found that this response was shown to be related to an increase of extracellular dopamine in the NAc (Wenkstern et al. 1993). Furthermore, copulation has shown to increase Fos-IR in the NAc of male rats (Robertson et al. 1991), just like estrous female odors did in both, the Shell and Core, whereas a neutral cue paired with copulation did as well, yet only in the Core (Kippin et al. 2003). Similarly, Lopez and Ettenberg (2002) also found a higher Fos-IR activation in the NAc of males exposed to an estrous female versus a nonestrous female. This effect was greater in sexually experienced males than in naïve ones. Therefore, a reduced Fos-IR in the NAc in males preexposed to UnScF and later on trained with ScF may suggest that the odor did not have a strong incentive value as it was for the ScF group, as it can be seen also through the absence of a CEP in both groups. It is believed this effect is driven by US-context associations that blocked the associability. Furthermore, the Fos-IR in the NAc of males preexposed to ScF and later on trained with UnScF provides further evidence for the differential patter of associability of neutral cues depending when they are associated with sexual reward. Namely, the odor incentive value still remains high, although preexposing an odor cue five times before training a CEP based on the same preexposed cue. Finally, as previously mentioned, lesions in this brain area increased the refractory period (Liu et al. 1998), while showing a higher Fos-IR in males trained to associate an olfactory cue with sexual reward (Kippin et al. 2003); an effect that corresponds with the general higher latency of ejaculation found in both group of males preexposed to a ScF and later on trained with UnScF.
VTA
As previously stated, the VTA is the source of the mesolimbic dopamine pathway, thought to control or mediate different appetitive behaviors and attention toward reward-related stimuli and their incentive salience (Berridge 2007). The VTA and the NAc are connected largely via dopamine neurons that terminate in the NAc and are activated mainly, but not exclusively, in response to reward-related cues (Berridge 2007; Pfaus 2009). Lesions to this brain area disrupt sexual behaviors and increase the duration of the post-ejaculatory interval, but not to abolish copulation (for review, see Hull et al. 2006), whereas electrophysiological stimulation facilitated copulatory behavior in the male rat (Markowsky and Hull 1995), an effect found to be dependent of which portion of the VTA was stimulated (Rodrígues-Manzo and Pellicer 2007). Fos-IR increases in the VTA of male rats in response to female estrous odors, but not to a conditioned neutral odor paired with sexual reward (Kippin et al. 2003). Therefore, a lower Fos-IR count in the males preexposed five times to ScF and later on trained with UnScF in comparison to the ScF control group may suggest a reduction in the incentive value attributed to the odor cue. Also, as seen with the NAc, there was no reduction in the Fos-IR count in the group of males preexposed to ScF and later on trained with UnScF, corroborating the differential pattern of association. This pattern of association depending on when the odor is paired with sexual reward, and thus having a differential patter of activation between brain areas, may provide further evidence for the notion of the mPOA as the main brain area that encodes the value of the reward, and the VTA–NAc as brain areas that encode for the incentive value of the reward (GR Quintana, M Birrel, S Marceau, N Kalantari, J Bowden, Y Bachoura, E Bourdas, V Lemay, C Mac Cionnaith, JG Pfaus, in prep.).
CeA
The amygdala and its functions have been well documented in the sexual behavior of the male rat (e.g., Swanson and Petrovich 1998). As an arrangement of different nuclei, the amygdala has been regarded as an integrative site between chemosensory, somatosensory, and hormonal cues, projecting to hypothalamic areas playing a role in learning, motivational states, and sexual behavior (Everitt, 1990). Several studies have been conducted exploring the role of the medial and basolateral subnuclei of the amygdala in the male sexual behavior (see Hull and Rodriguez-Manzo 2009), yet much less work has been done on the role of the CeA. GABA-like inmunoreactivity in the brain of monkeys revealed a very dense array of predominantly GABA neurons and projections to other brain areas (McDonald and Augustine 1993). CeA inputs come from several cortical, thalamic, and brainstem areas, including the prefrontal insular, temporal and olfactory cortical areas, caudal thalamus, as well as almost all other sub-nuclei of the amygdala (Swanson and Petrovich 1998). The CeA is involved in the modulation of conditioned fear (van de Kar and Blair 1999). For instance, a study done with male prairie voles indicated that there was an increase in Fos-IR in response to cohabitation with an unfamiliar unrelated male (Cushing et al. 2003). This response was explained in terms of an increased response of anxiety and stress, since a reduction in anxiety has been previously found due to lesions of the CeA, but not in the BLA of animals performing anxiety-like tasks (Möller et al. 1997). Therefore, a higher Fos-IR in males preexposed one time to ScF and later trained with UnScF than the ScF control group suggests an inhibitory activation in response to the odor, consistent with the absence of CEP and the completely different pattern found in the mPOA. Also, a higher Fos-IR was found in the UnScF control compared to the ScF control, also the opposite of what was found in the mPOA. Once again, further experiments are necessary to corroborate these speculations.
BLA
This brain region is another of several subnuclei of the amygdala and has been studied extensively in the of classical and operant fear conditioning (e.g., Fanselow and LeDoux 1999). The BLA sends direct projections to NAc that have been implicated in sexual incentives, yet not with copulation (Everitt et al. 1989). Lesion studies have shown to impair the operant response associated with sexual reward, yet copulation remained identical to control animals (Everitt et al. 1989). As previously mentioned, Fos-IR increased following the exposure of a sexually conditioned odor in the BLA of male rats (Kippin et al. 2003). Although none of the comparisons resulted in significant differences or meaningful effect sizes, there was a decrement in the Fos-IR pattern in all preexposed groups compared to the ScF control group in the BLA. It is uncertain to explain this general decrement in all groups taking into account the previous findings of other studies and the limited knowledge of this brain area in the context of sexual reward. However, a decrement in both 5t and 1t groups preexposed to UnScF and later on trained with ScF may contribute to explain the decrement in the same groups found in their NAc core (and perhaps NAc shell, as well), considering that the BLA sends direct glutamatergic projections to this brain region that facilitate motivated-behavioral responding (Stuber et al. 2011), like the odor cue is believed to modulate in these males. However, further replication on the differential general pattern of decrement of this region is needed to properly determine why preexposure to UnScF and not to ScF may have led to a decrement in Fos-IR in the NAc core and shell.
Taken together, the results of this study provide further emphasis on the role of first sexual experiences in the male rat, and how these modulate future sexual preferences. Particularly, five trials of preexposure hindered the display of a CEP for either female. Conversely, being preexposed once to a ScF, and later trained with UnScF developed a preference for the latter, whereas being preexposed once to the UnScF, rendered a similar result as being preexposed five times. Furthermore, the Fos-IR data also argue for a differential role on the associability of neutral cues paired with sexual reward depending on when these come together, either early or later on in sexual experience, in which different areas would code for different aspects of the CS processing. What is the extent of these effects on partner preference, how long do they last, or what constitute as early or late in sexual experience, requires further experimentation.
Acknowledgments
This research was funded by grants from the Canadian Institutes of Health Research (MOP-74563) to J.G.P., the Consejo Nacional de Ciencia y Tecnología (Chile) to G.R.Q., and Fonds Québec de la Reserche en Santé to the Center for Studies in Behavioral Neurobiology. The authors would like to thank Nada Hafez, Amanda Harris, Misha Jackson, Mojdeh Nasr, and Kerstin Wenzel for their valuable work in the data collection.
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.048090.118.
References
- Ågmo A, Berenfeld R. 1990. Reinforcing properties of ejaculation in the male rat: role of opioids and dopamine. Behav Neurosci 104: 177–182. [DOI] [PubMed] [Google Scholar]
- Bakker J, van Ophemert J, Slob AK. 1996. Sexual differentiation of odor and partner preference in the rat. Physiol Behav 60: 489–494. [DOI] [PubMed] [Google Scholar]
- Baum MJ, Everitt BJ. 1992. Increased expression of c-Fos in the medial preoptic area after mating in male rats: role of afferent inputs from the medial amygdala and midbrain central tegmental field. Neuroscience 50: 627–646. [DOI] [PubMed] [Google Scholar]
- Berridge KC. 2007. The debate over dopamine's role in reward: the case for incentive salience. Psychopharmacology 191: 391–431. [DOI] [PubMed] [Google Scholar]
- Bressler SC, Baum MJ. 1996. Sex comparison of neuronal Fos inmunoreactivity in the rat vomeronasal projectin circuit after chemosensory stimulation. Neuroscience 71: 1063–1072. [DOI] [PubMed] [Google Scholar]
- Cabilio S. 1996. Behavioral observation program [unpublished computer software]. Concordia University. [Google Scholar]
- Carr WJ, Krames L, Costanzo DJ. 1970. Previous sexual experience and olfactory preference for novel versus original sex partners in rats. J Comp Physiol Psychol 71: 216–222. [DOI] [PubMed] [Google Scholar]
- Carr WJ, Loeb L, Dissinger ML. 1965. Responses of rats to sex odors. J Comp Physiol Psychol 59: 370–377. [DOI] [PubMed] [Google Scholar]
- Carr WJ, Solberg B, Pfaffmann C. 1962. The olfactory threshold for estrous female urine in normal and castrated male rats. J Comp Physiol Psychol 55: 415–417. [DOI] [PubMed] [Google Scholar]
- Cook EW III, Modes RL, Lang PJ. 1986. Preparedness and phobias: effects of stimulus content on human visceral conditioning. J Abnormal Psychol 95: 195–207. [DOI] [PubMed] [Google Scholar]
- Clasen MM, Hempel BJ, Riley AL. 2017. Pre-exposure to cocaine or morphine attenuates taste avoidance conditioning in adolescent rats: drug specificity in the US pre-exposure effect. Dev Psychobiol 59: 486–494. [DOI] [PubMed] [Google Scholar]
- Cushing BS, Mogekwu N, Le WW, Hoffman G, Carter S. 2003. Cohabitation induced Fos inmunoreactivity in the monogamous prairie vole. Brain Res 965: 203–211. [DOI] [PubMed] [Google Scholar]
- Dominguez JM, Hull EM. 2005. Dopamine, the preoptic area, and male sexual behavior. Physiol Behav 86: 356–368. [DOI] [PubMed] [Google Scholar]
- Everitt BJ. 1990. Sexual motivation: a neural and behavioral analysis of the mechanisms underlying appetitive and copulatory responses of male rats. Neurosci Biobehav Rev 14: 217–232. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Cador M, Robbins TW. 1989. Interactions between the amygdala and ventral striatum in stimulus-reward associations: studies using a second-order schedule of sexual reinforcement. Neuroscience 30: 63–75. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, LeDoux JE. 1999. Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron 23: 229–232. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Josselyn SA, Anagnostaras SG, Kogan JH, Takahashi E, Silva AJ. 2004. Consolidation of CS and US representations in associative fear conditioning. Hippocampus 14: 557–569. [DOI] [PubMed] [Google Scholar]
- Garcia J, Koelling RA. 1966. Relation of cue to consequence in avoidance learning. Psychonomic Sci 4: 123–124. [Google Scholar]
- Gilman DP, Westbrook WH. 1978. Mating preference and sexual reinforcement in female rats. Physiol Behav 20: 11–14. [DOI] [PubMed] [Google Scholar]
- Graham JM, Desjardins C. 1980. Classical conditioning: induction of luteinizing hormone and testosterone secretion in anticipation of sexual activity. Science 210: 1039–1041. [DOI] [PubMed] [Google Scholar]
- Holloway KS, Domjan M. 1993. Sexual approach conditioning: unconditioned stimulus factors. J Exp Psychol Animal Behav Process 19: 38–46. [DOI] [PubMed] [Google Scholar]
- Hull EM, Rodríguez-Manzo G. 2009. Male sexual behavior. In Hormones, brain and behavior, 2nd ed (ed. Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT), Vol. 1, pp. 5–65. Academic Press, San Diego. [Google Scholar]
- Hull EM, Wood RI, McKenna K. 2006. The neurobiology of male sexual behavior. In Knobil and Neill's physiology of reproduction, 3rd ed (ed. Neill JD), Vol. 2, pp. 1729–1842. Academic Press, San Diego, CA. [Google Scholar]
- Ismail N, Girard-Bériault F, Nakanishi S, Pfaus JG. 2009. Naloxone, but not flupenthixol, disrupts the development of conditioned ejaculatory preference in the male rat. Behav Neurosci 123: 992–999. [DOI] [PubMed] [Google Scholar]
- Kagan J, Beach FA. 1953. Effect of early experience on mating behavior in male rats. J Comp Physiol Psychol 46: 204–208. [DOI] [PubMed] [Google Scholar]
- Kippin TE, Pfaus JG. 2001a. The development of olfactory conditioned ejaculatory preferences in the male rat I. Nature of the unconditioned stimulus. Behav Brain Res 73: 457–469. [DOI] [PubMed] [Google Scholar]
- Kippin TE, Pfaus JG. 2001b. The nature of the conditioned response mediating olfactory conditioned ejaculatory preference in the male rat. Behav Brain Res 122: 11–24. [DOI] [PubMed] [Google Scholar]
- Kippin TE, Talianakis S, Schattmann L, Bartholomew S, Pfaus JG. 1998. Olfactory conditioning of sexual behavior and mate selection in the male rat. J Comp Psychol 112: 389–399. [Google Scholar]
- Kippin TE, Samaha AN, Sotiropoulos V, Pfaus JG. 2001. The development of olfactory conditioned ejaculatory preferences in the male rat II. Parametric manipulation of conditioning session number and duration. Physiol Behav 73: 471–485. [DOI] [PubMed] [Google Scholar]
- Kippin TE, Cain SW, Pfaus JG. 2003. Estrous odors and sexually conditioned neutral odors activate separate neural pathways in the male rat. Neuroscience 117: 971–979. [DOI] [PubMed] [Google Scholar]
- Kippin TE, Sotiropoulos V, Badih J, Pfaus JG. 2004. Opposing roles of the nucleus accumbens and anterior lateral hypothalamus area in control of sexual behavior in the male rat. Eur J Neurosci. 19: 698–704. [DOI] [PubMed] [Google Scholar]
- Liu Y, Sachs BD, Salamone JD. 1998. Sexual behavior in male rats after radiofrequency or dopamine-depleting lesions in nucleus accumbens. Pharmacol Biochem Behav 60: 585–592. [DOI] [PubMed] [Google Scholar]
- Lopez HH, Ettenberg A. 2002. Exposure to female rats produces differences in c-fos induction between sexually-naïve and experienced male rats. Brain Res 947: 57–66. [DOI] [PubMed] [Google Scholar]
- Lubow RE, Moore AU. 1959. Latent inhibition: the effect of nonreinforced preexposure of the conditioned stimulus. J Comp Physiol Psychol 52: 415–419. [DOI] [PubMed] [Google Scholar]
- Lueckemann L, Bösche K, Engler H, Schwitalla JC, Hadamitzky M, Schedlowski M. 2016. Pre-exposure to the unconditioned or conditioned stimulus does not affect learned immunosuppression in rats. Brain Behav Immunol 51: 252–257. [DOI] [PubMed] [Google Scholar]
- Markowski VP, Hull EM. 1995. Cholecystokinin modulates mesolimbic dopaminergic influences on male rat copulatory behavior. Brain Res 699: 266–274. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Augustine JR. 1993. Localization of GABA-like inmunoreactivity in the monkey amygdala. Neuroscience 53: 281–294. [DOI] [PubMed] [Google Scholar]
- Mehrara BJ, Baum MJ. 1990. Naloxone disrupts the expression but not the acquisition by male rats of a conditioned place preference response for an oestrous female. Psychopharmacology 101: 118–125. [DOI] [PubMed] [Google Scholar]
- Meisel RD, Sachs BD. 1995. The physiology of male reproduction. In The physiology of reproduction (ed. Knobil E, Neil JD), Vol. 2, pp. 3–105. Raven, New York. [Google Scholar]
- Miller RG. 1966. Simultaneous statistical inference. Springer. [Google Scholar]
- Mis FW, Moore JH. 1973. Effect of preacquisition UCS exposure on classical conditioning of the rabbit's nictitating membrane response. Learn Motiv 4: 108–114. [Google Scholar]
- Möller C, Wiklund L, Sommer W, Thorsell A, Heilig M. 1997. Decreased experimental anxiety and voluntary ethanol consumption in rats following central but not basolateral amygdala lesions. Brain Res 760: 94–101. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. 1998. The rat brain. 4th ed Academic Press, California. [Google Scholar]
- Pfaus JG. 2009. Pathways of sexual desire. J Sex Med 6: 1506–1533. [DOI] [PubMed] [Google Scholar]
- Pfaus JG, Pinel JPJ. 1989. Alcohol inhibits and disinhibits sexual behavior in the male rat. Psychobiology 17: 195–201. [Google Scholar]
- Pfaus JG, Wilkins MF. 1995. A novel environment disrupts copulation in sexually naive but not experienced male rats: reversal with naloxone. Physiol Behav, 57: 1045–1049. [DOI] [PubMed] [Google Scholar]
- Pfaus JG, Mendelson SD, Phillips AG. 1990. A correlational and factor analysis of anticipatory and consummatory measures of sexual behavior in the male rat. Psychoneuroendocrinology 15: 329–340. [DOI] [PubMed] [Google Scholar]
- Pfaus JG, Kippin TE, Centeno S. 2001. Conditioning and sexual behavior: a review. Horm Behav 40: 291–321. [DOI] [PubMed] [Google Scholar]
- Pfaus JG, Kippin TE, Coria-Ávila G. 2003. What can animal models tell us about human sexual response? Annu Rev Sex Res 14: 1–63. [PubMed] [Google Scholar]
- Pfaus JG, Ismail N, Coria-Ávila GA. 2010. Sexual motivation. In Encyclopedia of behavioral neuroscience (ed. Koob GF, Le Moal M, Thompson RF), Vol. 3, pp. 201–209. Academic Press, Oxford. [Google Scholar]
- Pfaus JG, Kippin TE, Coria-Ávila GA, Gelez H, Alfonso VM, Ismail N, Parada M. 2012. Who, what, where, when (and maybe even why)? How the experience of sexual reward connects sexual desire, preference, and performance. Arch Sex Behav 41: 31–62. [DOI] [PubMed] [Google Scholar]
- Quintana GR, Jackson M, Nasr M, Pfaus JG. 2018. Effect of CS preexposure on the conditioned ejaculatory preference of the male rat: behavioral analyses and neural correlates. Learn Mem 25: 513–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randich A, LoLordo VM. 1979. Associative and nonassociative theories of UCS preexposure phenomenon. Implications for Pavlovian conditioning. Psychol Bull 86: 523–548. [PubMed] [Google Scholar]
- Rankin CH, Abrams T, Barry RJ, Bhatnagar S, Clayton D, Colombo J, Coppola G, Geyer MA, Glanzman DL, Marsland S, et al. 2009. Habituation revisited: an updated and revised description of the behavioral characteristics of habituation. Neurobiol Learn Mem 92: 135–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla R, Wagner A. 1972. A theory of Pavlovian conditioning: variations in the effectiveness of reinforcement and nonreinforcement. In Classical conditioning II: current theory and research (ed. Black AH, Prokasky WF), pp. 64–99. Appleton–Century–Crofts, New York. [Google Scholar]
- Robertson GS, Pfaus JG, Atkinson LJ, Matsumura H, Phillips AG, Fibiger HC. 1991. Sexual behavior increases c-fos expression in the forebrain of the male rat. Brain Res 564: 352–357. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Manzo G, Pellicer F. 2007. Electrical stimulation of the ventral tegmental area exerts opposite effects on male rat sexual behavior expression depending on the stimulated sub region. Behav Brain Res 179: 310–313. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Manzo G, Pellicer F, Larsson K, Fernandez-Guasti A. 2000. Stimulation of the medical preoptic area facilitates sexual behavior but does not reverse sexual satiation. Behav Neurosci 114: 553–560. [PubMed] [Google Scholar]
- Sachs BD. 1997. Erection evoked in male rats by airborne scent from estrous females. Physiol Behav 62: 921–924. [DOI] [PubMed] [Google Scholar]
- Sachs BD, Barfield RJ. 1976. Functional analysis of masculine copulatory behavior in the rat. Adv Study Behav 7: 91–154. [Google Scholar]
- Seligman M. 1970. On the generality of the laws of learning. Psychol Rev 77: 406–418. [Google Scholar]
- Stern J. 1970. Responses of male rats to sex odors. Physiol Behav 5: 519–524. [DOI] [PubMed] [Google Scholar]
- Stuber GD, Sparta DR, Stamatakis AM, van Leeuwen WA, Hardjoprajitno JE, Cho S, Tye KM, Kempadoo KA, Zhang F, Deisseroth K, et al. 2011. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature 475: 377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Petrovich GD. 1998. What is the amygdala? Trends Neurosci 21: 323–331. [DOI] [PubMed] [Google Scholar]
- Taylor JA. 1956. Level of conditioning and intensity of the adaptation stimulus. J Exp Psychol 51: 127–130. [DOI] [PubMed] [Google Scholar]
- Tenk CM, Wilson H, Zhang Q, Pitchers KK, Coolen L. 2009. Sexual reward in male rats: effects of sexual experience on conditioned place preference associated with ejaculations and intromissions. Horm Behav 55: 93–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Kar LD, Blair ML. 1999. Forebrain pathways mediating stress-induced hormone secretion. Front Neuroendocrinol 20: 1–48. [DOI] [PubMed] [Google Scholar]
- Wenkstern D, Pfaus JG, Fibiger HC. 1993. Dopamine transmission increases in the nucleus accumbens of male rats during their first exposure to sexually receptive female rats. Brain Res 618: 41–46. [DOI] [PubMed] [Google Scholar]
- Wood DA, Kosobud AE, Rebec GV. 2004. Nucleus accumbens single-unit activity freely behaving male rats during approach to novel and non-novel estrus. Neurosci Lett 368: 29–32. [DOI] [PubMed] [Google Scholar]
- Zamble E, Mitchell JB, Findlay H. 1986. Pavlovian conditioning of sexual arousal: parametric and background manipulations. J Exp Psychol Anim Behav Process 12: 403–411. [PubMed] [Google Scholar]