ABSTRACT
The oncometabolite, D-2-hydroxyglutarate, accumulates in various cancers because of acquired mutations in isocitrate dehydrogenase 1 & 2. Here, we describe a new mechanism for D-2-hydroxyglutarate accumulation in breast cancer. It involves c-Myc signaling and alcohol dehydrogenase, iron-containing protein 1 (ADHFE1) and leads to metabolic reprogramming, de-differentiation, and increased mammary tumorigenesis.
KEYWORDS: Breast Cancer, Metabolism, oncogene, 2-hydroxyglutarate, Myc signaling, Mechanisms of oncogenesis and tumor progression, Oncogenes and tumor suppressor genes, Tumor metabolism
During malignant transformation, signaling by the c-Myc/MYC proto-oncogene protein or the loss of the tumor suppressor, TP53 (also known as p53), can rewire metabolic pathways to support tumor growth and metastasis.1 We previously examined breast tumors and adjacent non-cancerous tissue from 67 patients and described the differential abundance of more than 200 metabolites.2 One of them was 2-hydroxyglutarate, which was elevated up to 100-fold in tumors that were primarily estrogen receptor-negative. This accumulation of 2-hydroxyglutarate was closely associated with the co-occurrence of a c-Myc signaling signature in the tumors. D-2-hydroxyglutarate is a oncometabolite that accumulates in gliomas, leukemias, and other cancers because of acquired gain-of-function mutations in either cytosolic isocitrate dehydrogenase (IDH1) or mitochondrial IDH2.3 However, our investigations did not detect these mutations in the studied breast tumors, indicating that an alternative mechanism must lead to increased 2-hydroxyglutarate in breast cancer.
Both D- and L-2-hydroxyglutarate are oncometabolites4,5 and their accumulation – much like the increase in succinate and fumarate because of loss-of-function mutations in the succinate and fumarate dehydrogenase genes – inhibits α-ketoglutarate-dependent dioxygenases. Inhibition of these enzymes leads to DNA and histone hypermethylation, cellular de-differentiation, and predisposition to cancer.6,7 Because the investigations by Terunuma et al.2 neither identified the enantiomeric form of 2-hydroxyglutarate or the cause for its accumulation, we continued to interrogate the relationship between c-Myc signaling and increased 2-hydroxyglutarate in breast tumors. In the follow-up study by Mishra et al.,8 we now show that human breast tumors produce D-2-hydroxyglutarate as the predominant enantiomer whereas increased L-2-hydroxyglutarate occurs in some tumors in association with lactate dehydrogenase B expression. Furthermore, we describe D-2-hydroxyglutarate-producing alcohol dehydrogenase, iron-containing protein 1 (ADHFE1) as a breast cancer oncoprotein that is associated with disease survival. We show that ADHFE1 promotes a reductive glutamine metabolism with increased D-2-hydroxyglutarate and mitochondrial reactive oxygen species (ROS) formation (Figure 1). This leads to cellular de-differentiation and enhanced epithelial-mesenchymal transition (EMT), thereby phenocopying alterations in IDH-mutant cancer cells with high D-2-hydroxyglutarate. We also show that D-2-hydroxyglutarate by itself induces EMT and alters metabolism in mammary epithelial cells, including upregulation of both reductive carboxylation of glutamine-derived α-ketoglutarate and mitochondrial ROS.
Figure 1.
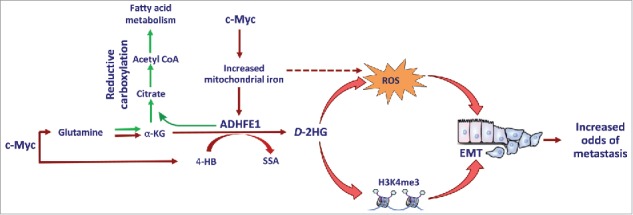
Upregulation of ADHFE1 promotes accumulation of D-2-hydroxyglutarate in breast tumors. c-Myc signaling increases D-2-hydroxyglutarate (D-2HG) by increasing the availability of both the substrates [4-hydroxybutyrate (4-HB) and α-ketoglutarate (α-KG)] and iron for the ADHFE1 enzyme. ADHFE1 and D-2HG up-regulate reductive carboxylation of glutamine-derived α-KG, reactive oxygen species (ROS) formation, and histone methylation at H3K4me3, leading to increased acetyl coenzyme A (acetyl CoA) and fatty acid synthesis and epithelial-mesenchymal transition (EMT). SSA, succinic semialdehyde; H3K4me3, histone trimethylation at histone H3, lysine 4.
We focused our investigations on ADHFE1 because ADHFE1 and MYC locus amplifications co-occur in breast tumors, as shown by Mishra et al..8 Additional data indicated the existence of a regulatory feedback loop between c-Myc signaling and ADHFE1 protein expression. The function of ADHFE1 as a candidate oncogene was corroborated when MCF7 human breast cancer cells overexpressing ADHFE1, MYC, or both ADHFE1 and MYC were injected into the mammary gland of mice. ADHFE1 and c-Myc expression not only enhanced tumor growth in a synergistic manner but also increased intratumor levels of 4HB and 2-hydroxyglutarate. 4HB is a substrate for ADHFE1 to produce D-2-hydroxyglutarate. c-Myc signaling can increase 4HB formation through upregulation of the enzyme, Aldo-Keto Reductase Family 7 Member A2, thereby enhancing D-2-hydroxyglutarate production in breast tumors. While Mishra et al. provided preliminary data to support this hypothesis, additional research is needed to elucidate the potentially oncogenic function of 4HB synthesis in breast cancer biology.
ADHFE1 enzymatic activity may have an important function in fatty acid metabolism. Its upregulation in MCF10A and MCF12A mammary epithelial cells induced prominent changes in metabolism including reductive carboxylation of α-ketoglutarate, which is a metabolic pathway that promotes fatty acid synthesis in the absence of oxygen. Accordingly, ADHFE1-overexpressing cells acquired a growth advantage when cultured under hypoxic conditions. ADHFE1 also induced a moderate but significant increase in mitochondrial ROS. The latter, but not reductive carboxylation of α-ketoglutarate, was found to be a key factor to the induction of EMT by ADHFE1. Currently, it appears that the accumulation of D-2-hydroxyglutarate accounts for most of the phenotypes associated with ADHFE1 (e.g., increased reductive carboxylation, ROS, and EMT). Yet, additional investigations should test whether ADHFE1 promotes oncogenic phenotypes independent of D-2-hydroxyglutarate.
ADHFE1 is an iron-sulfur enzyme and its expression depends on iron availability, as Mishra et al. showed. c-Myc is a key regulator of iron metabolism and up-regulates genes in this pathway.9 Since we did not find that c-Myc induces ADHFE1 through promoter activation, the effect of c-Myc on iron metabolism is likely the primary driver of c-Myc-induced ADHFE1. Moreover, an increased expression of iron metabolism genes has been reported for breast cancer cell lines with an aggressive mesenchymal phenotype,10 which tend to have aberrant c-Myc signaling. Little is known about the function of ADHFE1 in these cells. Future studies may test whether 2-hydroxyglutarate and ROS, together with increased iron, account for at least some of the aggressive biology of mesenchymal breast cancer cell lines. Another key finding of our study is the observation that ADHFE1 significantly upregulates intracellular D-2-hydroxyglutarate under hypoxic conditions. Because of the additional finding that ADHFE1-overexpressing cells efficiently secrete D-2-hydroxyglutarate into the extracellular space, we speculate that an increased ADHFE1 enzymatic activity may not only affect cell biology but also the tumor microenvironment through D-2-hydroxyglutarate-mediated effects, particularly under hypoxic conditions.
In conclusion, our study shows that human breast tumors produce oncogenic D-2-hydroxyglutarate using a distinct pathway that involves c-Myc signaling and the mitochondrial enzyme, ADHFE1 (Figure 1). The upregulation of ADHFE1 and D-2-hydroxyglutarate similarly induced EMT, stemness, and invasion of mammary cells through increased ROS and changes in histone and DNA methylation. Because 2-hydroxyglutarate accumulates more so in basal-like breast tumors than other breast cancer subtypes, as shown by Terunuma et al.,2 inhibition of ADHFE1 may offer a new therapeutic strategy for basal-like and triple-negative breast cancers.
Funding Statement
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Disclosure of potential conflict of interest
The authors declare no conflict of interest.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
References
- 1.Mishra P, Ambs S. Metabolic signatures of human breast cancer. Mol Cell Oncol. 2015;2:e992217. doi: 10.4161/23723556.2014.992217. PMID:26005711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Terunuma A, Putluri N, Mishra P, Mathe EA, Dorsey TH, Yi M, Wallace TA, Issaq HJ, Zhou M, Killian JK, et al.. MYC-driven accumulation of 2-hydroxyglutarate is associated with breast cancer prognosis. J Clin Invest. 2014;124:398–412. doi: 10.1172/JCI71180. PMID:24316975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ward PS, Patel J, Wise DR, bdel-Wahab O, Bennett BD, Coller HA, Cross JR, Fantin VR, Hedvat CV, Perl AE, et al.. The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting alpha-ketoglutarate to 2-hydroxyglutarate. Cancer Cell. 2010;17:225–34. doi: 10.1016/j.ccr.2010.01.020. PMID:20171147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Losman JA, Looper RE, Koivunen P, Lee S, Schneider RK, McMahon C, Cowley GS, Root DE, Ebert BL, Kaelin WG Jr.. (R)-2-hydroxyglutarate is sufficient to promote leukemogenesis and its effects are reversible. Science. 2013;339:1621–5. doi: 10.1126/science.1231677. PMID:23393090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shim EH, Livi CB, Rakheja D, Tan J, Benson D, Parekh V, Kho EY, Ghosh AP, Kirkman R, Velu S, et al.. L-2-Hydroxyglutarate: an epigenetic modifier and putative oncometabolite in renal cancer. Cancer Discov. 2014;4:1290–8. doi: 10.1158/2159-8290.CD-13-0696. PMID:25182153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu C, Ward PS, Kapoor GS, Rohle D, Turcan S, bdel-Wahab O, Edwards CR, Khanin R, Figueroa ME, Melnick A, et al.. IDH mutation impairs histone demethylation and results in a block to cell differentiation. Nature 2012;483:474–8. doi: 10.1038/nature10860. PMID:22343901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waterfall JJ, Killian JK, Meltzer PS. The role of mutation of metabolism-related genes in genomic hypermethylation. Biochem Biophys Res Commun. 2014;455:16–23. doi: 10.1016/j.bbrc.2014.08.003. PMID:25111818. [DOI] [PubMed] [Google Scholar]
- 8.Mishra P, Tang W, Putluri V, Dorsey TH, Jin F, Wang F, Zhu D, Amable L, Deng T, Zhang S, et al.. ADHFE1 is a breast cancer oncogene and induces metabolic reprogramming. J Clin Invest. 2018;128:323–340. doi: 10.1172/JCI93815; PMID: 29202474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu KJ, Polack A, Dalla-Favera R. Coordinated regulation of iron-controlling genes, H-ferritin and IRP2, by c-MYC. Science. 1999;283:676–9. doi: 10.1126/science.283.5402.676. PMID:9924025. [DOI] [PubMed] [Google Scholar]
- 10.Marques O, da Silva BM, Porto G, Lopes C. Iron homeostasis in breast cancer. Cancer Lett 2014;347:1–14. doi: 10.1016/j.canlet.2014.01.029. PMID:24486738. [DOI] [PubMed] [Google Scholar]


