Summary
Cytomegalovirus (CMV) infection is a potentially fatal complication in patients receiving haematopoietic stem cell transplantation (HSCT), but recent evidence indicates that CMV has strong anti‐leukaemia effects due in part to shifts in the composition of natural killer (NK) cell subsets. NK cells are the primary mediators of the anti‐leukaemia effect of allogeneic HSCT, and infusion of allogeneic NK cells has shown promise as a means of inducing remission and preventing relapse of several different haematological malignancies. The effectiveness of these treatments is limited, however, when tumours express human leucocyte antigen (HLA)‐E, a ligand for the inhibitory receptor NKG2A, which is expressed by the vast majority of post‐transplant reconstituted and ex‐vivo expanded NK cells. It is possible to enhance NK cell cytotoxicity against HLA‐Epos malignancies by increasing the proportion of NK cells expressing NKG2C (the activating receptor for HLA‐E) and lacking the corresponding inhibitory receptor NKG2A. The proportion of NKG2Cpos/NKG2Aneg NK cells is typically low in healthy adults, but it can be increased by CMV infection or ex‐vivo expansion of NK cells using HLA‐E‐transfected feeder cells and interleukin (IL)‐15. In this review, we will discuss the role of CMV‐driven NKG2Cpos/NKG2Aneg NK cell expansion on anti‐tumour cytotoxicity and disease progression in the context of haematological malignancies, and explore the possibility of harnessing NKG2Cpos/NKG2Aneg NK cells for cancer immunotherapy.
Keywords: immunotherapy, leukemia, myeloma, NK-cells, NKG2C
CMV and cancer: a double‐edged sword?
Cytomegalovirus (CMV) is a prevalent β‐herpesvirus that infects 50–80% of all adults in the United States 1. CMV is typically asymptomatic in immunocompetent hosts; however, it has been linked to increased risk of cancer‐related and post‐transplant mortality in haematopoietic stem cell transplantation (HSCT) and organ transplant recipients, respectively 2, 3. Moreover, research into immunological function and causes of death in elderly people have led to the conclusion that CMV is an immunological burden within the T cell compartment that impacts negatively upon both immune status and overall health 4. These findings caused some to speculate that it may be beneficial to eradicate CMV or vaccinate against it as a matter of interest to public health 5; however, recent evidence suggests that CMV may also play a beneficial role in ‘arming’ natural killer (NK) cells to destroy cancerous blood cells 6, 7. This is due, in part, to the fact that NK cells have evolved countermeasures that allow them to control viral replication by up‐regulating activating receptors and down‐regulating inhibitory receptors for human leucocyte antigen (HLA) expressed on the surface of virally infected cells 8, 9, 10, 11. For example, both acute and latent CMV infections trigger substantial expansions of NKG2Cpos/NKG2Aneg NK cells 9, 12, 13, 14, which enables the elimination and containment of CMV‐infected cells that up‐regulate HLA‐E as a means of evading detection by NKG2Apos NK cells 10. HLA‐E can signal through either the inhibitory receptor NKG2A or the activating receptor NKG2C; however, the inhibitory receptor is dominant, thus only NKG2Cpos/NKG2Aneg NK cells are able to effectively kill HLA‐Epos target cells 15, 16, 17. These NKG2Cpos/NKG2Aneg NK cells generate recall responses to CMV that are evocative of immunological memory 13, which has led some to refer to them as ‘adaptive’ or ‘memory‐like’ NK cells 18, 19. These memory‐like properties of NK cells were first demonstrated in mice when it was shown that Ly49Hpos NK cells (Ly49H is the functional counterpart to human NKG2C) were expanded preferentially in mice subjected to murine CMV infection and that these CMV‐induced Ly49Hpos NK cells were long‐lived and able to mount recall responses to subsequent CMV exposure 20. Recently, it has been shown that there are two distinct types of long‐lived NK cells in mice, one being antigen‐specific (memory) and the other being cytokine‐driven 21. Specifically, Ly49Hpos memory NK cells show enhanced effector functions and anti‐tumour cytotoxicity (similar to our findings with NKG2Cpos NK cells in humans) 8, while cytokine‐activated long‐lived NK cells (Ly49Hneg) have greater responsiveness to interleukin (IL)‐15 and persist longer in a CMV‐free milieu 21. Evidence has also been provided for immunological memory in human NK cells, as CMV has been shown to induce epigenetic and phenotypical changes to NK cells that are stable over many years 22, 23. Supporting further the notion of ‘memory’ NK cells in humans, NKG2Cpos NK cells protect against acute CMV infection in solid organ transplant recipients 20, and NKG2Cpos NK cells from CMVpos donors expand preferentially in response to CMV reactivation following allo‐HSCT, but NKG2Cpos NK cells from CMVneg donors do not 24. Moreover, expansion of NKG2Cpos FcRγ‐deficient NK cells can be driven by CMV‐specific antibodies, which suggests an additional adaptive component to this ‘memory’ response 19.
Up‐regulation of NKG2C on ‘adaptive’ or ‘memory‐like’ NK cells responding to CMV has been linked to decreased expression of the transcription factor promyelocytic leukaemia zinc finger (PLZF) and downstream hypomethylation (up‐regulation) of transcripts encoding NKG2C isoforms 18. These ‘adaptive’ NKG2Cpos NK cells have enhanced target‐specific cytotoxicity (antibody‐dependent and ‐independent) and production of interferon (IFN)‐γ and tumour necrosis factor (TNF)‐α 8, 25, but markedly reduced effector responses to the innate cytokines IL‐12 and IL‐18 18. Furthermore, hypermethylation (down‐regulation) of promoters for the transmembrane intracellular signalling proteins [Fc receptor common gamma chain (FcɛRγ)], spleen tyrosine kinase (SYK) and EWS/FLI1‐activated transcript 2 (EAT‐2) has also been observed in NKG2Cpos NK cells taken from CMVpos donors 18, 19 with many purported downstream effects, including enhanced antibody‐dependent cellular cytotoxicity (ADCC) 25, impaired innate production of immunoregulatory cytokines and reduced elimination of activated T cells (i.e. heightened viral‐specific T cell responses) 18. Furthermore, CMV‐specific antibody‐dependent expansion of FcRγ‐deficient NK cells 19 can augment further the proportion of NKG2Cpos NK cells, as lack of FcɛRγ correlates strongly with NKG2C expression 18.
While an increased proportion of NKG2Cpos NK cells has been reported to be protective against CMV reactivation 24, no direct link has been established between the NKG2C receptor and increased NK cell activation in response to CMV as NKG2Cpos NK cells have poor direct effector responses toward CMV‐infected cells 25, 26, 27. However, more recent studies have reported that NKG2Cpos NK cells have enhanced ADCC markedly against CMV‐infected cells 28, 29, due probably to down‐regulation of FcRγ 25. Thus, it may be that ADCC is required for NKG2Cpos NK cell‐mediated killing of CMV‐infected cells.
As with CMV infection, tumour escape has been linked to up‐regulation of HLA‐E on transformed cells 10, 30, which has led researchers to explore the role of CMV and NKG2Cpos NK cells on cancer prognosis and treatment response. Interestingly, it has been reported in multiple prospective cohort studies that reactivation of CMV post‐transplant is associated with a remarkable reduction in the risk of 100‐day relapse in acute myelogenous leukaemia (AML) 31, 32, 33, 34 and chronic myelogenous leukaemia (CML) 35 patients receiving allogeneic HSCT. The mechanism underpinning this beneficial effect of CMV reactivation on leukaemic relapse is currently unknown; however, we and others have hypothesized that the effect may be mediated by CMV‐induced shifts in the composition of NK cell subsets 33, 36. Our work suggests that this unknown mediating factor may be NKG2Cpos/NKG2Aneg NK cells. Specifically, we have shown that the CMV‐driven accumulation of NKG2Cpos/NKG2Aneg NK cells is associated with a strong anti‐leukaemia and anti‐myeloma effect that is commensurate with the magnitude of tumour cell HLA‐E expression 8, 37. Using an HLA‐E‐transfected 721.221 lymphoma cell line (221.AEH), we were able to show that the beneficial effect of CMV on anti‐tumour cytotoxicity was HLA‐E‐dependent, as CMV‐infected individuals showed greater NK cell activity against lymphoma cells constitutively expressing HLA‐E (221.AEH), but not HLA‐E void 721.221 cells 8. This effect was also shown to be NKG2C‐dependent, as antibody blockade of the NKG2C receptor eliminated the CMV effect on NK cell activity against 221.AEH cells, and it was also shown that it was only the NKG2Cpos NK cells that degranulated and produced IFN‐γ in response to 221.AEH cells 8. Furthermore, the increased cytotoxic activity of NK cells seen in people with CMV was replicated in CMV‐seronegative individuals by expanding NKG2Cpos/NKG2Aneg NK cells preferentially using 221.AEH feeder cells and IL‐15 in vitro 8. Liu et al. 38 recently corroborated our findings by showing that ex‐vivo expansion of NKG2Cpos NK cells using 221.AEH feeder cells enhances NK cell cytotoxicity against paediatric acute lymphoblastic leukaemia (ALL) blasts. Additional support for the important role of HLA‐E comes from Sarkar et al. 39, who reported that primary myeloma cells express heterogeneously high levels of HLA‐E and that HLA‐E inhibits stoichiometrically the anti‐myeloma cytotoxicity of NKG2Apos NK cells. Collectively, these data suggest that CMV infection might prime NK cells to recognize and destroy malignant HLA‐Epos cells through the accumulation of highly functional NKG2Cpos NK cells (see Fig. 1)
Figure 1.
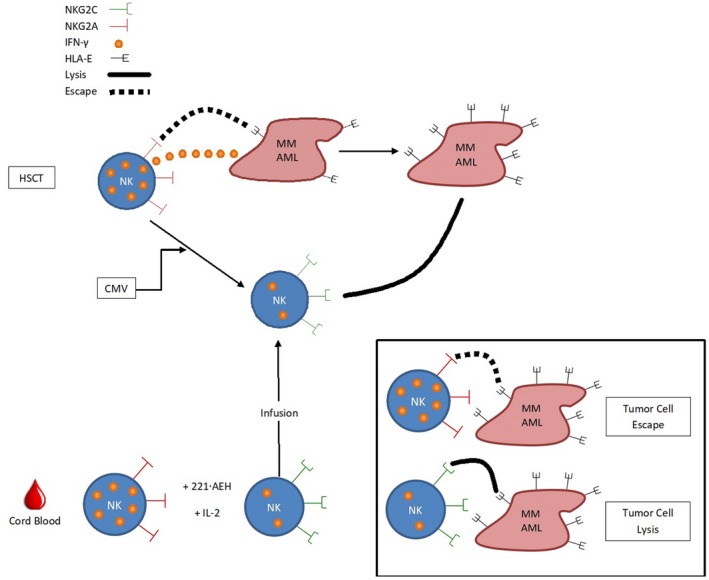
Cytomegalovirus (CMV)‐driven accumulation or ex‐vivo expansion of NKG2Cpos/NKG2Aneg natural killer (NK) cells enhances NK cell cytotoxicity against human leucocyte antigen (HLA)‐Epos tumour cells. HLA‐E signals through the activating receptor NKG2C and the inhibitory receptor NKG2A, with signalling through NKG2A being dominant, thus only NKG2Cpos/NKG2Aneg NK cells are able to effectively lyse HLA‐Epos target cells. NK cells are the first lymphocyte subset to recover following an allogeneic haematopoietic stem cell transplantation (HSCT), but the cells that are first to reconstitute are ∼80% NKG2Apos, which makes them unable to kill acute myeloid leukaemia (AML), multiple myeloma (MM) and other haematological malignancies characterized by high HLA‐E expression. Furthermore, these ‘naive’ NKG2Apos NK cells produce copious amounts of interferon (IFN)‐γ, which further up‐regulate HLA‐E expression by haematological tumour cells. CMV infection/reactivation increases the proportion of NKG2Cpos/NKG2Aneg NK cells, enhances cytotoxicity against HLA‐Epos tumour cells and reduces the risk of leukaemic relapse markedly. While CMV seropositivity and reactivation have been correlated positively with decreased risk of leukaemic relapse, this benefit is outweighed by increased non‐relapse mortality due to complications of CMV infection. To mimic the beneficial anti‐tumour effect of CMV without the negative consequences of CMV infection, we developed a protocol to mass produce NKG2Cpos/NKG2Aneg NK cells from peripheral or cord blood samples and enhance cytotoxicity against HLA‐Epos malignancies. Infusion of these ex‐vivo expanded NKG2Cpos NKcells may improve immunotherapy for the treatment of HLA‐Epos malignancies.
Recent clinical data also support this hypothesis 32, 40, as NKG2Cpos NK cells expand during CMV reactivation in allogeneic HSCT recipients 24 and leukaemic blasts, in turn, have a high expression of HLA‐E 41. In one major investigation, Cichocki et al. 31 showed prospectively in 674 allogeneic HSCT recipients that the expansion of highly differentiated (CD56dim/CD57pos) NKG2Cpos NK cells is associated with a decreased incidence of leukaemic relapse and increased disease‐free survival up to 1 year post‐transplant. This study reported a broadly beneficial effect of CMV reactivation on relapse risk for haematological malignancies, as the study included patients with AML (n = 313), ALL (n = 187), myelodysplastic syndrome (MDS) (n = 85), non‐Hodgkin lymphoma (NHL) (n = 42), CML (n = 28), Hodgkin's disease (n = 14) and multiple myeloma (MM) (n = 5) 31. Interestingly, in a smaller prospective study of 106 allogeneic HSCT patients afflicted with a variety of haematological malignancies (including 37 patients with AML, 21 with MDS and 12 with CLL), Bjorklund et al. 42 reported a protective effect of the naive NK cell repertoire (defined as NKG2Cneg/NKG2Apos/CD57neg) on leukaemic relapse. This study directly contradicts the findings of Cichocki et al. 31, as it reports a deleterious effect of increased NK cell differentiation (NKG2Cpos/CD56dim/CD57pos) on leukaemic relapse, disease‐free survival and overall survival 9–12 months after allogeneic HSCT 42. It is important to note when resolving the discrepancies between the two studies that the patients and donors were completely different. For example, the donors for the Bjorklund et al. 42 study were all adults, while the donor source for the Cichocki et al. 31 study was primarily cord blood 31, 42. In addition, all the patients in the Bjorklund et al. 42 study received myoablative (MA) conditioning, while the beneficial effect of CMV reported in the Cichocki et al. 31 paper was observed only in patients who received reduced intensity conditioning (RIC) 31, 42. Thus, the beneficial effect of CMV‐driven expansion of NKG2Cpos NK cells on treatment response in the Cichocki et al. 31 study may be at least partially attributable to residual host immunity (although 90% of NK cells were donor‐derived at 6 months) 31. Furthermore, monocyte concentrations were found to correlate strongly with NKG2Cpos NK cell expansion and these were higher in patients receiving RIC than in those conditioned with MA; thus, it could be that residual host monocytes are required for the beneficial effect of CMV and they may explain the difference between results obtained with RIC and MA conditioning 31. Conversely, the beneficial effect of a naive donor NK cell repertoire 42 may be attributable to lower expression of inhibitory killer‐cell immunoglobulin‐like receptor (KIR) for host HLA on the surface of immature NKG2Apos donor NK cells 42. Adding further complexity to the issue is the report by Manjappa et al. [43], that the beneficial effect of CMV reactivation on leukaemic relapse in allogeneic HSCT recipients is observed only in those receiving MA conditioning and not those receiving RIC (again contradicting Cichocki et al. 31). Use of common anti‐graft‐versus‐host disease (GVHD) prophylactic drugs such as anti‐thymocyte globulin has also been shown to blunt the anti‐leukaemia effect of CMV reactivation in allogeneic HSCT recipients 43. Moreover, Achour et al. 44 have reported that the incidence of head/neck and colorectal tumours is correlated positively with the expansion of NKG2Cpos NK cells in CMV‐infected liver transplant patients, which suggests that the effect of CMV and NKG2Cpos NK cells is actually negative with some categories of cancer 45. Thus, the field is currently beset with equivocal findings and future studies should seek to resolve the ambiguities and also determine if the benefits of CMV‐induced NK cell reconstitution on prognosis/relapse risk extend to HLA‐E‐expressing malignancies beyond leukaemia (e.g. MM) 39.
It is important to note that while donor CMV seropositivity 46 and CMV reactivation 32, 33 have been correlated positively with a decreased risk of leukaemic relapse in allogeneic HSCT recipients, this salubrious effect does not outweigh the risk of increased non‐relapse mortality that CMV carries 34, 46. Our work and that of others suggests that the benefit of CMV on the incidence of leukaemic relapse may be attributable to an elevated frequency of NKG2Cpos NK cells that are able to target HLA‐E‐expressing leukaemic blasts effectively 8, 31, 41. Thus, it follows that infusion of ex‐vivo expanded NKG2Cpos NK cells may allow us to simulate the beneficial effect of CMV on incidence of leukaemic relapse, while simultaneously reducing the risk of CMV reactivation 24, 47 and consequently reducing the risk of non‐relapse mortality in HSCT recipients. It is also plausible that the potential immunotherapeutic benefits of NKG2Cpos NK cells could be extended to other HLA‐E‐expressing cancers besides leukaemia 30, 39.
Harnessing the power of CMV: NKG2Cpos NK cells and immunotherapy
The idea of harnessing the power of NK cells for the treatment of cancer can be traced back to the landmark study by Ruggeri et al. 48, who showed that AML patients receiving an allogeneic HLA‐mismatched HSCT with KIR–ligand incompatibility had extremely favourable outcomes relative to those receiving transplants without it. Specifically, it was shown that KIR–ligand mismatch completely protected against haematopoietic graft rejection, acute GVHD and leukaemic relapse at 5 years (0 versus 75% in KIR‐matched donors), and that this effect was entirely attributable to anti‐recipient and anti‐leukaemia NK cell clones that arose post‐transplant 49. The promising results of Ruggeri et al. 48 led to the idea that adoptive transfer of NK cells may serve as an effective means of controlling AML in the absence of HSCT.
In one study, 19 patients with AML were infused with a single NK cell‐enriched product derived from a haploidentical‐related donor 50. Of the four patients receiving NK cells from KIR‐mismatched donors, three achieved a complete remission while only two of 15 patients receiving NK cells from KIR‐matched donors achieved remission. Other studies have shown beneficial effects of KIR–ligand mismatch on HSCT and cord blood transplantation in AML patients. For example, KIR–ligand mismatch was associated with a decreased incidence of relapse and improved survival in AML patients receiving an unrelated cord blood transplant 51, and the absence of one or more KIR ligands for donor inhibitory KIR was associated with improved survival and a decreased incidence of relapse in AML and MDS patients receiving T cell‐depleted (NK‐rich) HSCT from unrelated donors 52. While most studies reported a beneficial effect of KIR–ligand mismatch on treatment outcome (increased remission or decreased relapse) in leukaemia patients receiving either allogeneic NK cells directly or some form of allogeneic HSCT 50, 51, 52, 53, 54, 55, 56, some others reported no benefit 57, 58, 59, 60, and one even reported an increased incidence of relapse 61. Furthermore, a larger follow‐up study by the aforementioned Miller group demonstrating the potential to enhance allogeneic NK cell immunotherapy through selective depletion of regulatory T cells (Tregs) showed no correlation between KIR–ligand mismatch and remission rates in AML patients 62. Overall, however, the generally successful record of allogeneic, KIR‐mismatched NK cells in the prevention of relapse in leukaemia patients has led to attempts to utilize the technique to treat other haematological malignancies, such as MM 63, 64, 65.
High‐dose chemotherapy in combination with autologous HSCT is the standard treatment for MM 66, 67 and allogeneic HSCT is relatively rare 68, thus there are not the multitude of studies examining the potential anti‐myeloma activity of allogeneic NK cells that we see with leukaemia patients who are treated commonly with varying forms of allogeneic HSCT 69. However, given the fact that most MM patients fail to achieve a complete remission post‐transplant 70, new strategies are needed to improve treatment outcomes for MM, and adoptive transfer of allogeneic KIR‐mismatched NK cells is one avenue that has been explored with some clinical benefit being reported. For example, it has been shown that KIR–ligand mismatch in T cell‐depleted (NK cell‐enriched) allogeneic HSCT protects against relapse in MM patients 71 and infusion of haploidentical, KIR‐mismatched NK cells led to complete or near‐complete remission in 50% of patients with relapsed MM 65. While KIR–ligand mismatch has improved outcomes in allogeneic transplantation for MM, infusion of an adequate dose of alloreactive NK cells is difficult 65, which has led to the development of new techniques to achieve large clinical‐grade NK cell expansion 64, 72. Existing protocols generate exponentially large NK cell expansions 64, 72, but alloreactivity of donor NK cells is still highly variable 49, 65, 73 and expression of NKG2A is far greater than NKG2C 64, which limits the capacity of NK cells to kill myeloma cells with high HLA‐E expression 65, 73. This can be extremely important in the context of immunotherapy, as HLA‐E expression is up‐regulated on MM cells as the disease progresses 74 and HLA‐E can eliminate the beneficial effects of KIR–ligand mismatch by engaging NKG2A on NK cells 39. Moreover, NKG2Apos NK cells typically make up more than 80% of NK cells expanded from peripheral blood or stem cells 64, 75, 76 as well as reconstituted NK cells during the early post‐transplant period of allo‐HSCT 41. This inhibitory effect of HLA‐E on NKG2Apos NK cells may explain why infusion of KIR–ligand mismatched NK cells in relapsed myeloma patients produced a complete remission in only two of 10 patients 65. New protocols should exploit the immunoevasive strategies of MM to generate NK cells with high cytotoxicity against primary myeloma, especially those HLA‐Ebright cells which are particularly resistant to existing protocols that generate large numbers of NKG2Apos NK cells 64, 72. Such an approach would probably also be beneficial in the context of leukaemia, because AML and other leukaemic blasts are characterized by high HLA‐E expression 30, 77 due, in part, to up‐regulation of HLA‐E by IFN‐γ‐producing NKG2Apos NK cells, which make up the great majority of early post‐allogeneic HSCT NK cells 41. One such approach would be to mimic the effect of CMV on NK cells, as CMV has been shown to induce a marked expansion of NKG2Cpos/NKG2Aneg NK cells 12 which are, in turn, able to lyse HLA‐E expressing tumour cell lines that NKG2Apos NK cells cannot 8, 78.
To this end, our laboratory has shown that ex‐vivo expansion of NKG2Cpos/NKG2Aneg NK cells derived from fresh peripheral blood mononuclear cells (PBMCs) can be achieved using 221.AEH feeder cells (HLA‐E transfected) and IL‐15, with a resultant increase in NK cell activity against several distinct HLA‐Epos targets 8. Current expansion protocols rely on the proliferation‐inducing cytokines IL‐2/‐15 and transgenic feeder cell lines that constitutively express transmembrane pro‐growth cytokines such as IL‐15 and IL‐21, all of which enhance expression of NKG2A relative to NKG2C on stimulated NK cells 64, 79. By co‐culturing NK cells with the transgenic, HLA‐Ebright 221.AEH cell line, we mimic the effect of CMV and counter cytokine‐driven up‐regulation of NKG2A by preferentially activating and expanding NKG2Cpos/NKG2Aneg NK cells 8. Our approach can enhance the proportion of NKG2Cpos/NKG2Aneg NK cells from < 5% to greater than 50% when compared to conventional expansion techniques 8, 64. These NKG2Cpos/NKG2Aneg NK cells are able to recognize and eliminate CMV‐infected cells 12, 28, 29 and haematological malignancies that up‐regulate HLA‐E 8 as a means of immunoevasion. This enhanced anti‐tumour and anti‐viral cytotoxicity comes without damaging healthy tissue, as NKG2Cpos NK cells express self‐KIR 22 and healthy cells have lower expression of HLA‐E and lack the co‐stimulatory molecules required for full activation 80, 81. Furthermore, the cytotoxic effects of NKG2Cpos NK cells against HLA‐Epos malignancies do not require ADCC, which is necessary for NKG2Cpos NK cell‐mediated effector functions against CMV‐infected cells 8, 28, 29. It remains to be seen, however, if our approach for selectively expanding NKG2Cpos/NKG2Aneg NK cells can be combined with other clinical expansion protocols that generate higher NK cell yields (e.g. those using irradiated transgenic K562 cells) or if the final NK cell product will be effective in vivo or kill primary tumour cells as well as cell lines. If successful, the clinical grade ex‐vivo expansion of NKG2Cpos/NKG2Aneg NK cells may enhance the efficacy of NK cell‐based immunotherapy by enabling the production of highly cytotoxic ‘off‐the‐shelf’ NK cell lines for the treatment of MM, AML and other malignancies characterized by high HLA‐E expression (see Fig. 1).
While the idea of harnessing the anti‐tumour effects of CMV for NK cell immunotherapy is compelling, it is important to note that the effects of CMV on NK cell function are not universally beneficial. For example, Fielding et al. 82 report that members of the CMV US12 family of genes are able to down‐regulate expression of ligands for NK cell‐activating receptors on the surface of CMV‐infected targets. Specifically, CMV infection induces expression of US18 and US20 which synergistically suppress cell surface expression of B7‐H6 [82], a major ligand for the activating receptor NKp30 83. Additionally, CMV‐induced gpUL16 is able to sequester ligands for the activating receptor NKG2D 84. It is of note, however, that these immunoevasive strategies centre on protecting CMV‐infected cells from being recognized by NK cells rather than inhibiting NK cell function directly. Thus, NKG2C‐mediated killing of tumour targets by ex‐vivo expanded NKG2Cpos/NKG2Aneg NK cells would probably not be impaired by these CMV evasion strategies. Separate from our findings linking NKG2C with anti‐tumour activity, other activating receptors, such as NKG2D, NKp30 and activating KIR, have also been shown to drive cytotoxicity against haematological malignancies 49, 85. Besides increasing the proportion of NKG2Cpos NK cells, co‐culture of peripheral blood mononuclear cells with 221.AEH feeder cells can lead to an increased proportion of NK cells expressing activating KIR (similarly to CMV infection) 22. Thus, it is plausible that other activating receptors besides NKG2C will play a role in the anti‐tumour effect of the NKG2Cpos NK cell lines.
In addition to enhanced killing of HLA‐Epos malignancies, ex‐vivo expansion of NKG2Cpos NK cells has other benefits that could be harnessed for use in cancer immunotherapy. First, expansion of NKG2Cpos NK cells leads to skewing of the KIR repertoire towards self‐KIR 22 thus enhancing alloreactivity of NK cells against malignancies from HLA‐mismatched recipients (i.e. C2/C2 donors and C1/C1 recipients). For example, ex ‐vivo expansion of NKG2Cpos NK cells has been shown to enhance cytotoxicity against mismatched primary paediatric ALL blasts, an HLA‐E deficient malignancy, by enhancing the proportion of alloreactive NK cells 38. Secondly, NKG2Cpos NK cells are far more potent mediators of ADCC than their NKG2Cneg counterparts 86 with ADCC being one of the primary mechanisms whereby NKG2Cpos NK cells mediate effector responses in vivo 29. Thirdly, NKG2Cpos NK cells have been linked to the prevention of GVHD, as a low ratio of NKG2C to NKG2A on NK cells is associated with a marked increase in the risk of severe acute and chronic GVHD in HLA‐mismatched allogeneic HSCT recipients 87. Lastly, efficacy of NK cell immunotherapy protocols has been limited by poor persistence and loss of effector functions in transferred NK cells; however, NKG2Cpos NK cells have been reported to have ‘memory‐like’ persistence in vivo 19, 22. Collectively, these findings show that the benefits of ex‐vivo expansion of NKG2Cpos NK cells for cancer immunity go far beyond direct interaction of NKG2C with the HLA‐E receptor and that this approach can be combined effectively with other immunotherapeutic tools such as KIR–ligand mismatch and monoclonal antibody treatments (see Fig. 2).
Figure 2.
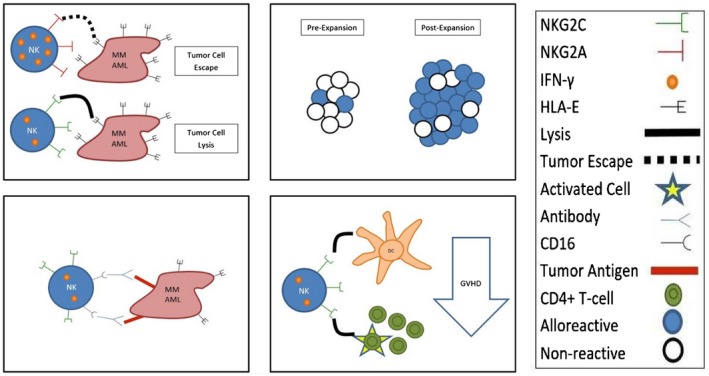
NKG2Cpos/NKG2Aneg natural killer (NK) cells have a broad range of benefits that can be harnessed effectively for cancer immunotherapy. (1) NKG2Cpos/NKG2Aneg NK cells are the only NK cells capable of efficiently lysing HLA‐Epos tumour cells. (2) The ex‐vivo expansion of NKG2Cpos/NKG2Aneg NK cells skews the killer cell immunoglobulin‐like receptor (KIR) repertoire towards self‐KIR, thus enhancing alloreactivity of allogeneic NK cells against malignancies from human leucocyte antigen (HLA)‐mismatched recipients. (3) NKG2Cpos NK cells are potent mediators of antibody‐dependent cell‐mediated cytotoxicity (ADCC), thus adoptive transfer of NKG2Cpos NK cells is expected to synergize well with monoclonal antibody‐based treatments. (4) NKG2Cpos/NKG2Aneg NK cells have strong anti‐graft‐versus‐host disease (GVHD) effects due in part to their ability to lyse residual host dendritic cells and activated CD4pos T cells. Thus, infusion of ex‐vivo expanded NKG2Cpos/NKG2Aneg NK cells could reduce incidence of both acute and chronic GVHD in the context of allogeneic haematopoietic stem cell transplantation (HSCT). Overall, adoptive transfer of NKG2Cpos/NKG2Aneg NK cells shows great promise as a tool for the treatment of a variety of haematological malignancies characterized by high HLA‐E expression, and the approach can be combined synergistically with other established techniques, including KIR/ligand mismatch and monoclonal antibody infusions.
Conclusions
CMV infection is a potentially fatal complication in patients receiving HSCT for the treatment of haematological malignancies, but recent evidence indicates that it may also have inadvertent salubrious effects. Specifically, CMV reactivation is associated with a marked reduction in the risk of relapse in leukaemia patients after allogeneic HSCT 32, 33. This anti‐leukaemia effect has been shown to correlate strongly with the expansion of NKG2Cpos NK cells 31, which are able to lyse leukaemic blasts that typically express constitutively high levels of HLA‐E 41. NK cells are a critical component of the anti‐leukaemia effect of allogeneic HSCT, and infusion of allogeneic NK cells has shown promise as a means of inducing remission in leukaemia and myeloma patients 50, 65 and preventing leukaemic relapse 88. Efficacy of these treatments is limited, however, by the difficulty of acquiring adequate numbers of alloreactive NK cells 65 and high expression of NKG2A relative to NKG2C in expanded NK cells 64. These limitations are particularly salient when dealing with haematological malignancies characterized by high expression of HLA‐E, as HLA‐Epos tumour cells can only be lysed by NKG2Cpos/NKG2Aneg NK cells 39. The proportion of NKG2Cpos/NKG2Aneg NK cells is typically low in healthy adults, but is elevated markedly in individuals infected with CMV 13, 22. We have shown in healthy donors that even latent CMV infection is associated with increased NK cell cytotoxic activity against a variety of haematological malignancies (leukaemia, MM and lymphoma), and that this effect is proportionate to the magnitude of target cell HLA‐E expression and is NKG2C‐dependent 8, 37. Furthermore, we demonstrated that this CMV effect could be mimicked in NK cells taken from CMV‐seronegative individuals by expanding NKG2Cpos/NKG2Aneg NK cells preferentially ex vivo using HLA‐E transfected feeder cells and IL‐15 8. From all the above, it is clear that NKG2Cpos/NKG2Aneg NK cells represent an intriguing immunotherapeutic target for the treatment of a variety of cancers characterized by high HLA‐E expression. Furthermore, NKG2Cpos/NKG2Aneg NK cells can be combined with other immunotherapy procedures, such as KIR–ligand mismatch and monoclonal antibody treatments to potentiate their effects. Future studies should explore the role of NKG2Cpos NK cells in the progression of other non‐leukaemic cancers and determine if NKG2Cpos/NKG2Aneg NK cells expanded ex vivo or taken directly from CMV‐infected individuals are able to kill primary tumour cells as effectively as cell lines 6, 8.
Disclosure
None.
Acknowledgements
This work was supported by an NIH grant R21 CA197527‐01A1 to A. B. B. and R. J. S.; and NASA grants NNX12AB48G and NNX16AB29G to R. J. S.
This article accompanies the following article: Bigley AB, Rezvani K, Shah N et al., Latent cytomegalovirus infection enhances anti‐tumour cytotoxicity through accumulation of NKG2C+ NK cells in healthy humans. Clin Exp Immunol, 185: 239‐251. doi:10.1111/cei.12785
References
- 1. Bate SL, Dollard SC, Cannon MJ. Cytomegalovirus seroprevalence in the United States: the national health and nutrition examination surveys, 1988–2004. Clin Infect Dis 2010; 50:1439–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Desai R, Collett D, Watson CJ, Johnson PJ, Moss P, Neuberger J. Impact of cytomegalovirus on long‐term mortality and cancer risk after organ transplantation. Transplantation 2015; 99:1989–94. [DOI] [PubMed] [Google Scholar]
- 3. Gkrania‐Klotsas E, Langenberg C, Sharp SJ, Luben R, Khaw KT, Wareham NJ. Seropositivity and higher immunoglobulin g antibody levels against cytomegalovirus are associated with mortality in the population‐based European prospective investigation of Cancer‐Norfolk cohort. Clin Infect Dis 2013; 56:1421–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sansoni P, Vescovini R, Fagnoni FF et al New advances in CMV and immunosenescence. Exp Gerontol 2014; 55:54–62. [DOI] [PubMed] [Google Scholar]
- 5. Pawelec G. Immunosenescence: role of cytomegalovirus. Exp Gerontol 2014; 54:1–5. [DOI] [PubMed] [Google Scholar]
- 6. Bigley AB, Spielmann G, Agha N, O'Connor DP, Simpson RJ. Dichotomous effects of latent CMV infection on the phenotype and functional properties of CD8+ T‐cells and NK cells. Cell Immunol 2016; 300:26–32. [DOI] [PubMed] [Google Scholar]
- 7. White DW, Keppel CR, Schneider SE et al Latent herpesvirus infection arms NK cells. Blood 2010; 115:4377–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bigley AB, Rezvani K, Shah N et al Latent cytomegalovirus infection enhances anti‐tumour cytotoxicity through accumulation of NKG2C+ NK cells in healthy humans. Clin Exp Immunol 2016; 185:239–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature 2009; 457:557–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tomasec P, Braud VM, Rickards C et al Surface expression of HLA‐E, an inhibitor of natural killer cells, enhanced by human cytomegalovirus gpUL40. Science 2000; 287:1031. [DOI] [PubMed] [Google Scholar]
- 11. Vales‐Gomez M, Reyburn HT, Erskine RA, Lopez‐Botet M, Strominger JL. Kinetics and peptide dependency of the binding of the inhibitory NK receptor CD94/NKG2‐A and the activating receptor CD94/NKG2‐C to HLA‐E. Embo J 1999; 18:4250–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Guma M, Angulo A, Vilches C, Gomez‐Lozano N, Malats N, Lopez‐Botet M. Imprint of human cytomegalovirus infection on the NK cell receptor repertoire. Blood 2004; 104:3664–71. [DOI] [PubMed] [Google Scholar]
- 13. Lopez‐Verges S, Milush JM, Schwartz BS et al Expansion of a unique CD57(+)NKG2Chi natural killer cell subset during acute human cytomegalovirus infection. Proc Natl Acad Sci USA 2011; 108:14725–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Monsivais‐Urenda A, Noyola‐Cherpitel D, Hernandez‐Salinas A et al Influence of human cytomegalovirus infection on the NK cell receptor repertoire in children. Eur J Immunol 2010; 40:1418–27. [DOI] [PubMed] [Google Scholar]
- 15. Borrego F, Masilamani M, Marusina AI, Tang X, Coligan JE. The CD94/NKG2 family of receptors: from molecules and cells to clinical relevance. Immunol Res 2006; 35:263–78. [DOI] [PubMed] [Google Scholar]
- 16. Colonna M, Moretta A, Vely F, Vivier E. A high‐resolution view of NK cell receptors: structure and function. Immunol Today 2000; 21:428–31. [DOI] [PubMed] [Google Scholar]
- 17. Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol 2008; 9:495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schlums H, Cichocki F, Tesi B et al Cytomegalovirus infection drives adaptive epigenetic diversification of NK cells with altered signaling and effector function. Immunity 2015; 42:443–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee J, Zhang T, Hwang I et al Epigenetic modification and antibody‐dependent expansion of memory‐like NK cells in human cytomegalovirus‐infected individuals. Immunity 2015; 42:431–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sun JC, Lopez‐Verges S, Kim CC, DeRisi JL, Lanier LL. NK cells and immune ‘memory’. JImmunol 2011; 186:1891–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nabekura T, Lanier LL. Tracking the fate of antigen‐specific versus cytokine‐activated natural killer cells after cytomegalovirus infection. JExp Med 2016; 213:2745–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Beziat V, Liu LL, Malmberg JA et al NK cell responses to cytomegalovirus infection lead to stable imprints in the human KIR repertoire and involve activating KIRs. Blood 2013; 121:2678–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fehniger TA, Cooper MA. Harnessing NK cell memory for cancer immunotherapy. Trends Immunol 2016; 37:877–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Foley B, Cooley S, Verneris MR et al Human cytomegalovirus (CMV)‐induced memory‐like NKG2C(+) NK cells are transplantable and expand in vivo in response to recipient CMV antigen. JImmunol 2012; 189:5082–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang T, Scott JM, Hwang I, Kim S. Antibody‐dependent memory‐like NK cells distinguished by FcRγ‐deficiency. JImmunol 2013; 190:1402–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Magri G, Muntasell A, Romo N et al NKp46 and DNAM‐1 NK cell receptors drive the response to human cytomegalovirus‐infected myeloid dendritic cells overcoming viral immune evasion strategies. Blood 2011; 117:848–56. [DOI] [PubMed] [Google Scholar]
- 27. Petersen L, Petersen CC, Moller‐Larsen A, Hokland ME. Short‐term exposure to human cytomegalovirus‐infected fibroblasts induces a proportional increase of active CD94/NKG2A(+) natural killer cells. Hum Immunol 2010; 71:29–35. [DOI] [PubMed] [Google Scholar]
- 28. Costa‐Garcia M, Vera A, Moraru M, Vilches C, López‐Botet M, Muntasell A. Antibody‐mediated response of NKG2Cbright NK cells against human cytomegalovirus. JImmunol 2015; 194:2715–24. [DOI] [PubMed] [Google Scholar]
- 29. Wu Z, Sinzger C, Frascaroli G et al Human cytomegalovirus‐induced NKG2C(hi) CD57(hi) natural killer cells are effectors dependent on humoral antiviral immunity. JVirol 2013; 87:7717–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lo Monaco E, Tremante E, Cerboni C et al Human leukocyte antigen E contributes to protect tumor cells from lysis by natural killer cells. Neoplasia 2011; 13:822–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cichocki F, Cooley S, Davis Z et al CD56dimCD57+NKG2C+ NK cell expansion is associated with reduced leukaemia relapse after reduced intensity HCT. Leukaemia 2016; 30:456–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Elmaagacli AH, Steckel NK, Koldehoff M et al Early human cytomegalovirus replication after transplantation is associated with a decreased relapse risk: evidence for a putative virus‐versus‐leukaemia effect in acute myeloid leukaemia patients. Blood 2011; 118:1402–12. [DOI] [PubMed] [Google Scholar]
- 33. Green ML, Leisenring WM, Xie H et al CMV reactivation after allogeneic HCT and relapse risk: evidence for early protection in acute myeloid leukaemia. Blood 2013; 122:1316–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Takenaka K, Nishida T, Asano‐Mori Y et al Cytomegalovirus reactivation after allogeneic hematopoietic stem cell transplantation is associated with a reduced risk of relapse in patients with acute myeloid leukaemia who survived to day 100 after transplantation: the Japan Society for Hematopoietic Cell Transplantation Transplantation‐related Complication Working Group. Biol Blood Marrow Transplant 2015; 21:2008–16. [DOI] [PubMed] [Google Scholar]
- 35. Ito S, Pophali P, Co W et al CMV reactivation is associated with a lower incidence of relapse after allo‐SCT for CML. Bone Marrow Transplant 2013; 48:1313–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bigley AB, Simpson RJ. NK cells and exercise: implications for cancer immunotherapy and survivorship. Discov Med 2015; 19:433–45. [PubMed] [Google Scholar]
- 37. Bigley AB, Rezvani K, Pistillo M et al Acute exercise preferentially redeploys NK cells with a highly‐differentiated phenotype and augments cytotoxicity against lymphoma and multiple myeloma target cells. Part II: impact of latent cytomegalovirus infection and catecholamine sensitivity. Brain Behav Immun 2015; 49:59–65. [DOI] [PubMed] [Google Scholar]
- 38. Liu LL, Beziat V, Oei VYS et al Ex vivo expanded adaptive NK cells effectively kill primary acute lymphoblastic leukaemia cells. Cancer Immunol Res 2017; 5:654–65. [DOI] [PubMed] [Google Scholar]
- 39. Sarkar S, van Gelder M, Noort W et al Optimal selection of natural killer cells to kill myeloma: the role of HLA‐E and NKG2A. Cancer Immunol Immunother 2015; 64:951–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Beck JC, Wagner JE, DeFor TE et al Impact of cytomegalovirus (CMV) reactivation after umbilical cord blood transplantation. Biol Blood Marrow Transplant 2010; 16:215–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nguyen S, Dhedin N, Vernant JP et al NK cell reconstitution after haploidentical hematopoietic stem‐cell transplantations: immaturity of NK cells and inhibitory effect of NKG2A override GvL effect. Blood 2005; 105:4135–42. [DOI] [PubMed] [Google Scholar]
- 42. Bjorklund AT, Clancy T, Goodridge JP et al Naive donor NK cell repertoires associated with less leukaemia relapse after allogeneic hematopoietic stem cell transplantation. JImmunol 2016; 196:1400–11. [DOI] [PubMed] [Google Scholar]
- 43. Manjappa S, Bhamidipati PK, Stokerl‐Goldstein KE et al Protective effect of CMV reactivation on relapse after allogeneic hematopoietic cell transplantation in AML patients is influenced by their conditioning regimen. Biol Blood Marrow Transplant 2014; 20:46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Busca A, Passera R, Pini M et al The use of ATG abrogates the antileukemic effect of cytomegalovirus reactivation in patients with acute myeloid leukaemia receiving grafts from unrelated donors. Am J Hematol 2015; 90:E117–21. [DOI] [PubMed] [Google Scholar]
- 45. Achour A, Baychelier F, Besson C et al Expansion of CMV‐mediated NKG2C+ NK cells associates with the development of specific de novo malignancies in liver‐transplanted patients. JImmunol 2014; 192:503–11. [DOI] [PubMed] [Google Scholar]
- 46. Nachbaur D, Clausen J, Kircher B. Donor cytomegalovirus seropositivity and the risk of leukemic relapse after reduced‐intensity transplants. Eur J Haematol 2006; 76:414–9. [DOI] [PubMed] [Google Scholar]
- 47. Foley B, Cooley S, Verneris MR et al Cytomegalovirus reactivation after allogeneic transplantation promotes a lasting increase in educated NKG2C+ natural killer cells with potent function. Blood 2012; 119:2665–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ruggeri L, Capanni M, Mancusi A et al Role of natural killer cell alloreactivity in HLA‐mismatched hematopoietic stem cell transplantation. Blood 2004; 33:216–339. [PubMed] [Google Scholar]
- 49. Ruggeri L, Capanni M, Urbani E et al Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 2002; 295:2097–100. [DOI] [PubMed] [Google Scholar]
- 50. Miller JS, Soignier Y, Panoskaltsis‐Mortari A et al Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood 2005; 105:3051–7. [DOI] [PubMed] [Google Scholar]
- 51. Willemze R, Rodrigues CA, Labopin M et al KIR‐ligand incompatibility in the graft‐versus‐host direction improves outcomes after umbilical cord blood transplantation for acute leukaemia. Leukaemia 2009; 23:492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hsu KC, Malkki M, Gooley TA, Dupont B, Petersdorf EW. Missing killer immunoglobulin‐like receptor (KIR) ligand confers protection from relapse in recipients of unrelated hematopoietic cell transplantation (HCT) for AML. Biol Blood Marrow Transplant 2005; 11:29. [Google Scholar]
- 53. Beelen DW, Ottinger HD, Ferencik S et al Genotypic inhibitory killer immunoglobulin‐like receptor ligand incompatibility enhances the long‐term antileukemic effect of unmodified allogeneic hematopoietic stem cell transplantation in patients with myeloid leukaemias. Blood 2005; 105:2594–600. [DOI] [PubMed] [Google Scholar]
- 54. Giebel S, Locatelli F, Lamparelli T et al Survival advantage with KIR ligand incompatibility in hematopoietic stem cell transplantation from unrelated donors. Blood 2003; 102:814–9. [DOI] [PubMed] [Google Scholar]
- 55. Miller JS, Cooley S, Parham P et al Missing KIR ligands are associated with less relapse and increased graft‐versus‐host disease (GVHD) following unrelated donor allogeneic HCT. Blood 2007; 109:5058–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Schaffer M, Malmberg K‐J, Ringden, O , Ljunggren H‐G, Remberger M. Increased infection‐related mortality in KIR‐ligand‐mismatched unrelated allogeneic hematopoietic stem‐cell transplantation. Transplantation 2004; 78:1081–5. [DOI] [PubMed] [Google Scholar]
- 57. Brunstein CG, Wagner JE, Weisdorf DJ et al Negative effect of KIR alloreactivity in recipients of umbilical cord blood transplant depends on transplantation conditioning intensity. Blood 2009; 113:5628–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chen C, Busson M, Rocha V et al Activating KIR genes are associated with CMV reactivation and survival after non‐T‐cell depleted HLA‐identical sibling bone marrow transplantation for malignant disorders. Bone Marrow Transplant 2006; 38:437–44. [DOI] [PubMed] [Google Scholar]
- 59. Davies SM, Ruggieri L, DeFor T et al Evaluation of KIR ligand incompatibility in mismatched unrelated donor hematopoietic transplants. Killer immunoglobulin‐like receptor. Blood 2002; 100:3825–7. [DOI] [PubMed] [Google Scholar]
- 60. Farag SS, Bacigalupo A, Eapen M et al The effect of KIR ligand incompatibility on the outcome of unrelated donor transplantation: a report from the center for international blood and marrow transplant research, the European blood and marrow transplant registry, and the Dutch registry. Biol Blood Marrow Transplant 2006; 12:876–84. [DOI] [PubMed] [Google Scholar]
- 61. Bornhauser M, Schwerdtfeger R, Martin H, Frank KH, Theuser C, Ehninger G. Role of KIR ligand incompatibility in hematopoietic stem cell transplantation using unrelated donors. Blood 2004; 103:2860–1. [DOI] [PubMed] [Google Scholar]
- 62. Bachanova V, Cooley S, Defor TE et al Clearance of acute myeloid leukaemia by haploidentical natural killer cells is improved using IL‐2 diphtheria toxin fusion protein. Blood 2014; 123:3855–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Garg TK, Szmania SM, Khan JA et al Highly activated and expanded natural killer cells for multiple myeloma immunotherapy. Haematologica 2012; 97:1348–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Shah N, Martin‐Antonio B, Yang H et al Antigen presenting cell‐mediated expansion of human umbilical cord blood yields log‐scale expansion of natural killer cells with anti‐myeloma activity. PLOS ONE 2013; 8:e76781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Shi J, Tricot G, Szmania S et al Infusion of haplo‐identical killer immunoglobulin‐like receptor ligand mismatched NK cells for relapsed myeloma in the setting of autologous stem cell transplantation. Br J Haematol 2008; 143:641–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Attal M, Harousseau JL, Facon T et al Single versus double autologous stem‐cell transplantation for multiple myeloma. N Engl J Med 2003; 349:2495–502. [DOI] [PubMed] [Google Scholar]
- 67. Attal M, Harousseau JL, Stoppa AM et al A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med 1996; 335:91–7. [DOI] [PubMed] [Google Scholar]
- 68. Koehne G, Giralt S. Allogeneic hematopoietic stem cell transplantation for multiple myeloma: curative but not the standard of care. Curr Opin Oncol 2012; 24:720–6. [DOI] [PubMed] [Google Scholar]
- 69. Koreth J, Schlenk R, Kopecky KJ et al Allogeneic stem cell transplantation for acute myeloid leukaemia in first complete remission: systematic review and meta‐analysis of prospective clinical trials. JAMA 2009; 301:2349–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Krejci M, Scudla V, Tothova E et al Long‐term outcomes of autologous transplantation in multiple myeloma: significant survival benefit of novel drugs in post‐transplantation relapse. Clin Lymphoma Myeloma 2009; 9:436–42. [DOI] [PubMed] [Google Scholar]
- 71. Kroger N, Shaw B, Iacobelli S et al Comparison between antithymocyte globulin and alemtuzumab and the possible impact of KIR‐ligand mismatch after dose‐reduced conditioning and unrelated stem cell transplantation in patients with multiple myeloma. Br J Haematol 2005; 129:631–43. [DOI] [PubMed] [Google Scholar]
- 72. Fujisaki H, Kakuda H, Shimasaki N et al Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer Res 2009; 69:4010–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Pende D, Spaggiari GM, Marcenaro S et al Analysis of the receptor‐ligand interactions in the natural killer‐mediated lysis of freshly isolated myeloid or lymphoblastic leukaemias: evidence for the involvement of the Poliovirus receptor (CD155) and Nectin‐2 (CD112). Blood 2005; 105:2066–73. [DOI] [PubMed] [Google Scholar]
- 74. Carbone E, Neri P, Mesuraca M et al HLA class I, NKG2D, and natural cytotoxicity receptors regulate multiple myeloma cell recognition by natural killer cells. Blood 2005; 105:251–8. [DOI] [PubMed] [Google Scholar]
- 75. Berg M, Lundqvist A, McCoy P Jr et al Clinical‐grade ex vivo‐expanded human natural killer cells up‐regulate activating receptors and death receptor ligands and have enhanced cytolytic activity against tumor cells. Cytotherapy 2009; 11:341–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Knorr DA, Ni Z, Hermanson D et al Clinical‐scale derivation of natural killer cells from human pluripotent stem cells for cancer therapy. Stem Cells Transl Med 2013; 2:274–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Nguyen S, Beziat V, Dhedin N et al HLA‐E upregulation on IFN‐gamma‐activated AML blasts impairs CD94/NKG2A‐dependent NK cytolysis after haplo‐mismatched hematopoietic SCT. Bone Marrow Transplant 2009; 43:693–9. [DOI] [PubMed] [Google Scholar]
- 78. Beziat V, Descours B, Parizot C, Debre P, Vieillard V. NK cell terminal differentiation: correlated stepwise decrease of NKG2A and acquisition of KIRs. PLOS ONE 2010; 5:e11966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Phan MT, Lee SH, Kim SK, Cho D. Expansion of NK cells using genetically engineered K562 feeder cells. Methods Mol Biol 2016; 1441:167–74. [DOI] [PubMed] [Google Scholar]
- 80. Guma M, Busch LK, Salazar‐Fontana LI et al The CD94/NKG2C killer lectin‐like receptor constitutes an alternative activation pathway for a subset of CD8+ T cells. Eur J Immunol 2005; 35:2071–80. [DOI] [PubMed] [Google Scholar]
- 81. López‐Botet M, Bellón T, Llano M, Navarro F, García P, de Miguel M. Paired inhibitory and triggering NK cell receptors for HLA class I molecules. Hum Immunol 2000; 61:7–17. [DOI] [PubMed] [Google Scholar]
- 82. Fielding CA, Weekes MP, Nobre LV et al Control of immune ligands by members of a cytomegalovirus gene expansion suppresses natural killer cell activation. Elife 2017; 10:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Brandt CS, Baratin M, Yi EC et al The B7 family member B7‐H6 is a tumor cell ligand for the activating natural killer cell receptor NKp30 in humans. JExp Med 2009; 206:1495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Rolle A, Mousavi‐Jazi M, Eriksson M et al Effects of human cytomegalovirus infection on ligands for the activating NKG2D receptor of NK cells: up‐regulation of UL16‐binding protein (ULBP)1 and ULBP2 is counteracted by the viral UL16 protein. JImmunol 2003; 171:902–8. [DOI] [PubMed] [Google Scholar]
- 85. Pogge von Strandmann E, Simhadri VR, von Tresckow B et al Human leukocyte antigen‐B‐associated transcript 3 is released from tumor cells and engages the NKp30 receptor on natural killer cells. Immunity 2007; 27:965–74. [DOI] [PubMed] [Google Scholar]
- 86. Beziat V, Dalgard O, Asselah T et al CMV drives clonal expansion of NKG2C+ NK cells expressing self‐specific KIRs in chronic hepatitis patients. Eur J Immunol 2012; 42:447–57. [DOI] [PubMed] [Google Scholar]
- 87. Kordelas L, Steckel NK, Horn PA, Beelen DW, Rebmann V. The activating NKG2C receptor is significantly reduced in NK cells after allogeneic stem cell transplantation in patients with severe graft‐versus‐host disease. Int J Mol Sci 2016; 17:1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Palmer JM, Rajasekaran K, Thakar MS, Malarkannan S. Clinical relevance of natural killer cells following hematopoietic stem cell transplantation. JCancer 2013; 4:25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]


