Abstract
In this work, Ni(I) aryl species that are directly relevant to cross-coupling have been synthesized. Transmetalation of (dppf)NiIX (dppf = 1,1′-bis(diphenylphosphino)-ferrocene, X = Cl, Br) with aryl Grignard reagents or aryl boronic acids in the presence of base produces Ni(I) aryl species of the form (dppf)NiI(Ar) (Ar = Ph, o-tolyl, 2,6-xylyl, 2,4,6-mesityl, 2,4,6-iPr3C6H2). The stability of the Ni(I) aryl species is inversely correlated to the steric bulk on the aryl ligand. The most unstable Ni(I) aryl species are the most active precatalysts for Suzuki–Miyaura reactions because they rapidly decompose to generate the active Ni(0) catalyst. This study shows that Ni(I) aryl species are initially formed in the activation of Ni(I) halide precatalysts for Suzuki–Miyaura reactions and establishes their stoichiometric and catalytic reactivity profile.
Keywords: nickel, cross-coupling, mechanism, homogeneous catalysis, inorganic synthesis
Graphical abstract
INTRODUCTION
There is significant current interest in the replacement of precious-metal catalysts with systems based on inexpensive and sustainable earth-abundant metals such as Ni.1 A major challenge in the development of Ni catalysts for applications such as cross-coupling2 is the propensity for these systems to readily access odd-electron oxidation states such as Ni(I).3 In some reactions such as Ni/photoredox dual catalysis,4 and alkyl–alkyl5 and cross-electrophile6 couplings, Ni(I) species are proposed to be catalytically active and facilitate novel reactivity. In contrast, in other reactions such as Suzuki–Miyaura7 and Buchwald–Hartwig8 couplings involving aryl substrates, as well as trifluoromethylation reactions,9 Ni(I) complexes are proposed to be off-cycle species. In both cases, our lack of fundamental knowledge about the reactivity and stability of Ni(I) species complicates the rational design of improved catalysts for variety of reactions including cross-coupling by promoting or inhibiting the formation of Ni(I) complexes.10–13
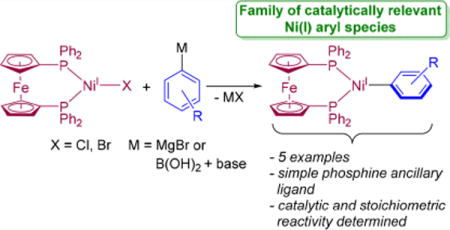
One important class of Ni(I) complexes are Ni(I) aryl species, which have been proposed to be present in many Ni-catalyzed cross-coupling reactions. For example, in Suzuki–Miyaura,7,11d Buchwald–Hartwig,11e and Kumada11d couplings, Ni(I) aryl species are hypothesized to form through transmetalation from Ni(I) halide species or through comproportionation reactions between catalytically active Ni(0) and Ni(II) aryl species. Additionally, in reactions related to cross-coupling, Ni(I) aryl complexes are postulated as intermediates in the carboxylation of aryl halides,11f,g triflates,11p and pivalates,11j and in the reductive cleavage of aryl ethers with silanes.11h However, despite their widely presumed existence in catalytic cycles, there have been almost no direct experimental studies exploring the role of Ni(I) aryl species in cross-coupling reactions. In fact, there are only two examples of well-characterized Ni(I) aryl species and in both cases the unusual steric or electronic properties of the aryl ligand limits their applicability to species likely to be generated in catalysis (Figure 1).14 Accordingly, there is almost no fundamental information about the formation and properties of Ni(I) aryl species.
Figure 1.
Summary of previous examples of Ni(I) aryl species and this work.
Previously, we explored the speciation of dppf-ligated (dppf = 1,1′-bis(diphenylphosphino)ferrocene) Ni precatalysts in Suzuki–Miyaura reactions. We found that, regardless of the starting oxidation state of the precatalyst, Ni(I) species are formed during catalysis.7,11l Although like others, we postulated that Ni(I) aryl species were formed in catalysis, and there was no direct evidence to support this hypothesis. Here, the preparation of a catalytically relevant family of dppf-ligated Ni(I) aryl species is described. We provide information about how these species are formed under catalytic conditions and their stability, stoichiometric and catalytic activity, and decomposition pathways. Further, it is demonstrated that Ni(I) halide precatalysts activate through the formation of Ni(I) aryl species, which decompose to catalytically active Ni(0) species.
RESULTS AND DISCUSSION
Synthesis and Properties of Nickel(I) Aryl Species
Past attempts to prepare Ni(I) aryl species via transmetalation of Ni(I) halide species with aryl Grignard reagents resulted in intractable mixtures of products with no evidence for the formation of the desired complex.11d,12d Consistent with these results, the reaction of (dppf)NiICl (1) or (dppf)NiIBr (2) with excess phenylmagnesium bromide in C6D6 at room temperature resulted in the rapid formation of an unidentified black precipitate. In contrast, the reaction of 1 or 2 with 2,4,6-triisopropylphenylmagnesium bromide resulted in the immediate appearance of identical signals from a paramagnetic molecule in the 1H NMR spectrum, which are assigned to the Ni(I) aryl complex (dppf)NiI(2,4,6-iPr3C6H2) (3). Even though 3 is only metastable in solution (vide infra), isolation was possible through the reaction of 1 with excess 2,4,6-triisopropylphenylmagnesium bromide at room temperature in 10:1 pentane:THF. The EPR spectrum of 3 is rhombic (gx = 2.03, gy = 2.15, and gz = 2.28) with hyperfine coupling to the phosphorus nuclei (see SI). The solid-state structure of 3 shows a distorted trigonal planar geometry around Ni (Figure 2), with a dppf bite angle of 103.430(19)° and P–Ni–C angles of 134.15(5)° and 122.42(5)°. The Ni–C bond length of 1.9762(17) Å is similar to those reported in the other two examples of Ni(I) aryl complexes, but direct comparison is difficult as one system is a two-coordinate complex with a sterically bulky aryl group (Ni–C = 1.944(2) Å),14a and the other contains a perfluorinated aryl group (Ni–C = 1.973(2) Å).14b
Figure 2.
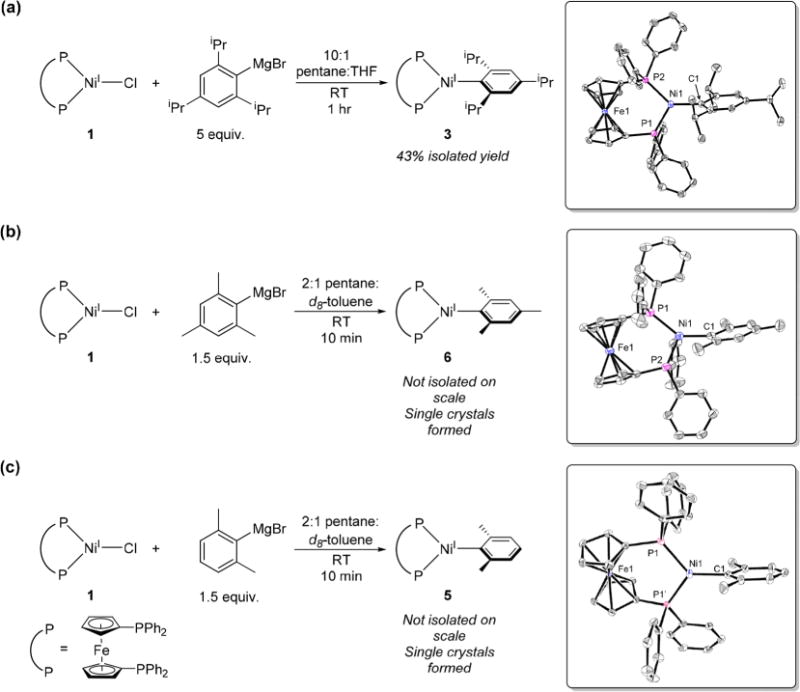
(a) Synthesis of 3. Inset: ORTEP of 3 with ellipsoids drawn at 50% probability. Hydrogen atoms and solvent of recrystallization are omitted for clarity. (b) and (c) Reactions of 1 with 2,4,6-mesitylmagnesium bromide or 2,6-xylylmagnesium bromide to generate 6 or 5, respectively, in situ. Insets: ORTEP of 6 or 5 with ellipsoids drawn at 50% probability. Hydrogen atoms and solvent of recrystallization is omitted for clarity. For 5, disorder in the xylyl ring is omitted for clarity.
The effect of steric bulk on the formation of Ni(I) aryl species was probed through the reactions of 1 or 2 with o-tolyl-, 2,6-xylyl- and 2,4,6-mesityl-magnesium bromide. In all cases, new paramagnetic signals were present in the 1H NMR spectra, analogous to those observed in the corresponding reaction between 2 and 2,4,6-triisopropylphenylmagnesium bromide (see SI). On this basis, the new signals are assigned to (dppf)NiI(o-tolyl) (4), (dppf)NiI(2,6-xylyl) (5), and (dppf)-NiI(2,4,6-mesityl) (6). As the size of the aryl group decreases, the stability of the Ni(I) aryl species decreases significantly, with 4 decomposing completely in approximately 6 h at room temperature. The instability of these species prevented isolation; however, single crystals of 5 and 6 were obtained using a related synthetic procedure to that described for 3 (Figure 2b,c). The solid-state structures of 5 and 6 are similar to 3, but the Ni–C bond lengths of 1.954(6) Å and 1.948(2) Å, respectively, are shorter, presumably due to the decreased size of the aryl group.
EPR spectroscopy provided additional evidence for the in situ formation of 4, 5, and 6. The low-temperature (7 K), X-band EPR spectra obtained from treatment of 2 with excess o-tolyl-, 2,6-xylyl-, or 2,4,6-mesityl-magnesium bromide at room temperature in toluene showed similar features to the spectrum of 3 (Figure 3). Further, when 2 was treated with phenyl-magnesium bromide at −90 °C in toluene and the reaction mixture frozen after 1 min, a signal was obtained using EPR spectroscopy. We propose that this signal is from (dppf)-NiI(Ph) (7), which is too unstable to observe at higher temperature. Comparison of the EPR spectra of 3–7 shows that complexes with relatively small aryl groups (4 and 7) can be accurately modeled as axial species with hyperfine contributions from two increasingly inequivalent phosphorus nuclei (see SI). In contrast, the complexes with larger aryl groups (3, 5, and 6) are best modeled as rhombic species. This suggests that increased steric bulk on the aryl group causes a distortion of the molecule and a lowering of the symmetry. Overall, our synthetic results conclusively establish that it is possible to form nickel(I) aryl species with a catalytically relevant ligand set and aryl groups.
Figure 3.
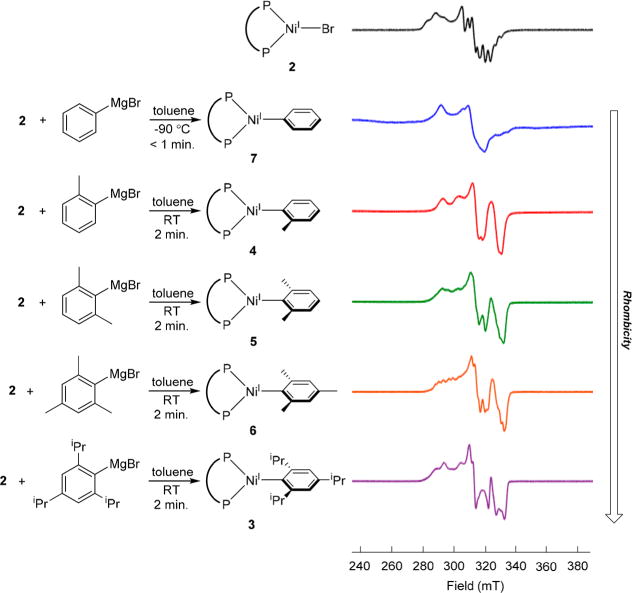
Low temperature (7 K) X-band EPR spectra in toluene glass of Ni(I) aryl species formed from the reaction of 2 with Grignard reagents at room temperature. Samples were frozen after 2 min of reaction in all cases except 7, which was prepared at −90 °C and frozen after 1 min. The spectrum of 2 (black, top) is provided for comparison. Detailed EPR parameters are provided in the SI.
The decomposition of the different Ni(I) aryl complexes was compared. When 3 is left to stand for 5 days at room temperature in C6D6, there is a 22% decrease in the concentration of the Ni(I) aryl species (see SI). The major organic product is 1,3,5-triisopropylbenzene,13e but no new Ni-containing products were observed by NMR spectroscopy. Monitoring the decomposition of 3 in the presence of 1,5-cyclooctadiene (COD) allowed identification of a Ni product. In this case, after 5 days at room temperature, there is 29% decomposition of 3, and (dppf)Ni0(COD) (8) (29% yield) and 1,3,5-triisopropylbenzene (29% yield) are observed. This suggests that 3 decomposes to a coordinatively unsaturated Ni(0) complex, which is trapped with COD. Notably, no biaryl products are observed when monitoring the decomposition of 3. However, when Ni(I) aryl species with smaller aryl groups, 4–6, are generated in situ, monoaryl and biaryl decomposition products are observed (see SI). One explanation for our observations is that the biaryl products arise from a decomposition pathway involving two Ni centers,15 whereas the monoaryl products involve a single Ni center. The sterically large aryl group on 3 could inhibit these pathways involving two Ni centers leading to only monoaryl products. Experiments examining the rate of decomposition as a function of the Ni(I) aryl concentration support this hypothesis. Specifically, changing the concentration of (dppf)NiI(2,4,6-iPr3C6H2) does not result in an observable change in the rate of decomposition, whereas more concentrated solutions of in situ generated (dppf)NiI(o-tolyl) lead to an increase in the rate of decomposition. Results from catalytic Suzuki–Miyaura reactions, where the concentration of Ni is low, are also consistent with this proposal, as the biaryl products proposed to arise from bimolecular degradation of putative Ni(I) aryl complexes are seen in smaller amounts than the monoaryl products.7
Stoichiometric Reactivity of Nickel(I) Aryl Species
Using the isolated Ni(I) aryl species 3 as a model, stoichiometric reactions were performed. Treatment of 3 with 2,6-lutidinium chloride at room temperature results in complete conversion to 1 over 1 h, with concomitant formation of 1,3,5-triisopropylbenzene (Figure 4a). In contrast, performing the reaction with 2,6-lutidinium tetrakis(3,5-bis(trifluoromethyl)-phenyl)borate (BArF4) generates a number of unidentified diamagnetic and paramagnetic species in addition to 1,3,5-triisopropylbenzene (see SI). In this reaction, the BArF4 anion presumably does not stabilize the nickel(I) species that forms after protonation, resulting in rapid decomposition to a mixture of products. Interestingly, 3 also reacts with sources of H• as well as H+. Reaction of 3 with TEMPOH (TEMPOH = 2,2,6,6,-tetramethyl-1-piperidinol) in the presence of COD as a Ni(0) trapping agent in C6D6 results in the degradation of 68% of the Ni(I) aryl species over 24 h at room temperature (Figure 4b). 1,3,5-Triisopropylbenzene is formed as an organic byproduct in 55% yield, along with the Ni(0) product 8 (61% yield). Additionally, the TEMPO radical was identified using room temperature X-band EPR spectroscopy (see SI). These results suggest that the Ni-aryl bond can be formally cleaved homolytically, resulting in H• transfer from TEMPOH to the aryl ligand and a reduced Ni(0) center that is trapped by COD, even though the exact mechanistic pathway for this reaction is unclear.
Figure 4.
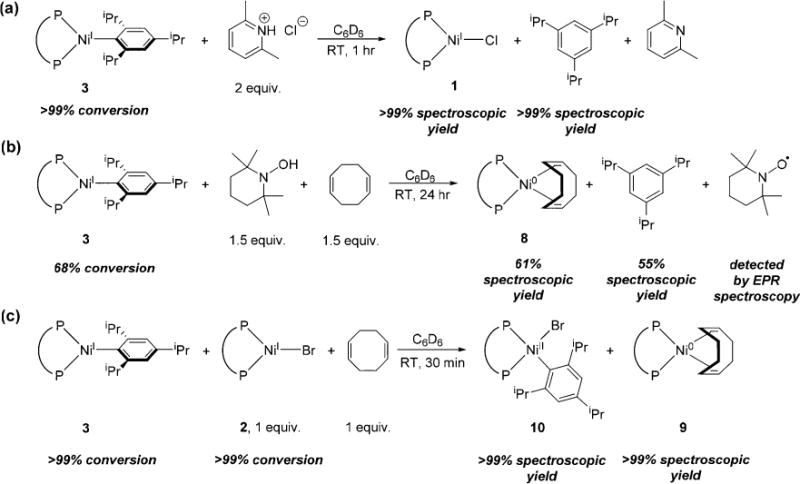
Stoichiometric reactivity of 3 with (a) 2,6-lutidinium chloride, (b) TEMPOH in the presence of COD, and (c) 2 in the presence of COD.
Although we have no evidence that 3 disproportionates into Ni(0) and Ni(II) complexes by itself, treatment of one equivalent of 3 with one equivalent of 2 in the presence of COD results in full conversion to (dppf)NiII(2,4,6-iPr3C6H2)-(Br) (9) (see SI for characterization) and 8 (Figure 4c). The reverse process, comproportionation, can be modeled by the reaction of 9 with a Ni(0) source, (dppf)Ni0(C2H4) (11). Consistent with our observation of complete disproportionation from 3 and 2 (in the presence of COD), we see no evidence for the reverse comproportionation reaction. This finding may be important for the rational design of improved Ni(II) precatalysts, as an analogous comproportionation reaction between Ni(0) and Ni(II) aryl halides with less sterically bulky aryl groups is proposed to produce off-cycle species in Suzuki–Miyaura reactions and reduce catalyst efficiency (see SI).7
The Role of Nickel(I) Aryl Species in Suzuki–Miyaura Reactions Using Nickel(I) Halide Precatalysts
It has previously been demonstrated that Ni(I) halide precatalysts are active for Suzuki–Miyaura and Kumada couplings involving aryl halides.7,11c,d,l Given that 1 and 2 do not react with excess aryl halide, even at elevated temperature,7,11l transmetalation is likely the first step in precatalyst activation. Our experiments above show that transmetalation is feasible in Kumada couplings, however, it is unclear if transmetalation is possible with weaker nucleophiles such as boronic acids. Treatment of 1 with excess 2,6-xylylboronic acid or 2,4,6-mesitylboronic acid in the presence of K3PO4 in C6D6 results in new paramagnetic resonances in the 1H NMR spectra at room temperature (Figure 5). These resonances have the same chemical shifts as those generated through the reactions of 1 or 2 with 2,6-xylyl- and 2,4,6-mesityl-magnesium bromide, indicating that 5 and 6 are formed (see SI). The transmetalation reactions are slower using boronic acids and base compared with Grignard reagents, and even after 24 h some starting material is still present. Nevertheless, the rate of transmetalation is comparable to the rate of product formation in Suzuki–Miyaura reactions with Ni(I) halide precatalysts.7
Figure 5.
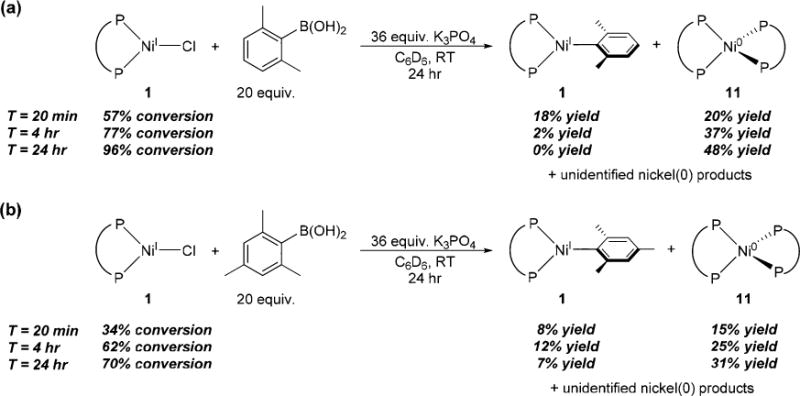
Reaction of 1 with (a) excess 2,6-xylylboronic acid and K3PO4, and (b) excess 2,4,6-mesitylboronic acid and K3PO4.
When 5 and 6 are generated via transmetalation with Grignard reagents, it takes approximately 2 days in solution at room temperature for complete decomposition to occur. In contrast, these species are less stable when generated using the appropriate boronic acid and K3PO4 and decay relatively rapidly to the homoleptic Ni(0) species (dppf)2Ni0 (11), as indicated by 31P NMR spectroscopy, and an unidentified product. The generation of 11 requires two equivalents of starting material, and therefore the maximum yield of 11 is 50%. The rapid formation of these zerovalent species suggests that boronic acid and base increase the rate of decomposition of Ni(I) aryl complexes. Consistent with this hypothesis, treatment of isolated 3 with excess o-tolylboronic acid and K3PO4 at room temperature results in essentially complete decomposition of the Ni(I) aryl species in 6 h (see SI). The increased rate of decomposition of Ni(I) aryl species in the presence of boronic acid and base may explain why they have not been observed in situ in Suzuki–Miyaura reactions where Ni(I) halide species were identified.7,11l Further, the observed decomposition of Ni(I) aryl species to 11 provides an explanation for the catalytic activity of Ni(I) halide precatalysts in Suzuki–Miyaura reactions, as 11 is known to be a highly active precatalyst.7,11l
On the basis of the results above, we propose that in Suzuki–Miyaura reactions using 1 as a precatalyst, generation of the catalytically active species occurs via initial transmetalation to form a Ni(I) aryl species. The Ni(I) aryl species could subsequently (i) undergo decomposition to form a catalytically active Ni(0) species (vide supra), or (ii) directly react with the electrophile. The preparation of Ni(I) aryl species allows us to examine the viability of pathway (ii). Addition of one equivalent of 1-chloronaphthalene to 3 in C6D6 did not result in any consumption of the electrophile. After 24 h at room temperature, the decrease in the amount of 3 was the same as when no electrophile was present, consistent with no reaction with electrophile occurring (see SI). Similar results were observed when 4 and 5 were generated in situ and treated with 1-chloronaphthalene. On this basis, it is proposed that pathway (i) is more likely, which indicates that even when Ni(I) halide precatalysts are used in Suzuki–Miyaura reactions, the catalytic cycle ultimately involves Ni(0) and Ni(II) complexes. These results also imply that Ni(I) aryl complexes are off-cycle species in Suzuki–Miyaura reactions (vide infra and see SI).
Nickel(I) Aryl Species as Precatalysts for Suzuki–Miyaura Reactions
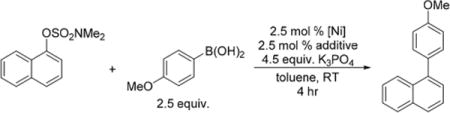
Based on our knowledge that Ni(I) aryl species decompose into Ni(0) species, and that some of these zerovalent species are catalytically competent, we hypothesized that pretreatment of Ni(I) halide precatalysts with Grignard reagents in situ could result in improved catalytic activity for Suzuki–Miyaura reactions. By itself, 1 cannot couple naphthalen-1-yl sulfamate with 4-methoxyphenylboronic acid at room temperature over 4 h (Table 1, entry 1). However, premixing 1 with aryl Grignard reagents to form Ni(I) aryl species, followed by addition to a mixture containing electrophile, boronic acid and base resulted in conversion to the coupled product. The yield of product is inversely correlated to the stability of the Ni(I) aryl complex, which is consistent with more rapid generation of Ni(0) giving improved catalytic activity. The most stable Ni(I) aryl species, 3, formed in situ by mixing 1 with 2,4,6-triisopropylphenyl-magnesium bromide, only gave a 19% yield of cross-coupled product (Table 1, entry 6), while the relatively unstable 4 reached full conversion (Table 1, entry 3). The only exception to this trend was the yield of cross-coupled product from the reaction catalyzed by in situ generated 7. In this case, the Ni aryl complex is so unstable (vide supra) that significant decomposition to a black precipitate occurred before mixing with the substrates, even when 7 was generated at low temperature (see SI). Notably, the catalytic activity of in situ generated 4 is comparable to the best Ni(0) and Ni(II) precatalysts for this reaction.7 When isolated 3 was used as the precatalyst a low yield (10%) of cross-coupled product was obtained, presumably due to the relative stability of 3 (Table 1, entry 7). Improved activity (76% yield) was obtained when a mixture containing a 1:1 ratio of TEMPOH and 3 was premixed and then added to a solution containing the substrates and base (Table 1, entry 8). We attribute this improvement in performance to the increased rate of formation of zerovalent Ni in the presence of H• donors, which provides further evidence in support of zerovalent Ni complexes being the true active species. A comparative selectivity experiment in which aryl sulfamate electrophiles with different electronic properties were coupled in a Suzuki–Miyaura reaction shows that 3, as well as Ni(I) halides mixed with different aryl Grignard reagents, give the same selectivity as a related Ni(0) precatalyst, providing more evidence that all systems react through the same active species (see SI). This experiment also provides further support to the hypothesis that Ni(I) aryl complexes are intermediates in the activation of Ni(I) halide precatalysts.
Table 1.
Comparison of Ni(I) Precatalysts for the Suzuki–Miyaura Reaction of Naphthalen-1-yl Sulfamate and 4-Methoxyphenylboronic Acida
| |||
|---|---|---|---|
| entry | [Ni] | additive | yield |
| 1 | 1 | none | <1% |
| 2 | 1 | (Ph)MgBr | 70% |
| 3 | 1 | (o-tolyl)MgBr | >99% |
| 4 | 1 | (2,6-xylyl)MgBr | 79% |
| 5 | 1 | (2,4,6-mesityl)MgBr | 79% |
| 6 | 1 | (2,4,6-iPr3C6H2)MgBr | 19% |
| 7 | 3 | none | 10% |
| 8 | 3 | TEMPOH | 76% |
Reaction conditions: 0.133 mmol naphthalene-1-yl dimethylsulfamate, 0.333 mmol 4-methoxyphenylboronic acid, 0.599 mmol K3PO4, 0.0665 mmol naphthalene (internal standard), 2.5 mol % precatalyst and 2.5 mol % additive, and 1 mL of toluene. Nickel precatalyst was premixed with additive prior to addition of other reagents (see SI). Yields are the average of two runs and were determined by GC.
CONCLUSIONS
We have synthesized a family of Ni(I) aryl species with catalytically relevant ligands and demonstrated that Ni(I) aryl species can be generated under conditions relevant to cross-coupling. Based on our results that Ni(I) aryl species can be formed via transmetalation, we propose that Ni(I) halide precatalysts activate through this pathway in catalysis. Additionally, in the Suzuki–Miyaura reactions studied in this work, Ni(I) aryl complexes are likely unstable off-cycle species that undergo decomposition to generate catalytically active Ni(0) complexes; thus, it is proposed that Ni(I) and Ni(0) precatalysts generate the same active species. The rate of decomposition of the Ni(I) aryl complexes increases as the size of the aryl substituent decreases, and they are less likely to form in comproportionation reactions between Ni(II) aryl halide complexes and Ni(0) complexes when the aryl group is large. We intend to utilize this latter observation to design improved Ni(II) precatalysts for Suzuki–Miyaura reactions, where the formation of off-cycle Ni(I) complexes is detrimental to catalysis. Our studies on well-defined Ni(I) aryl species may also assist in the improvement of many other catalytic reactions where these elusive complexes are implicated as either on-cycle or off-cycle species.
Supplementary Material
Acknowledgments
N.H. acknowledges support from the NIHGMS under Award Number R01GM120162. M.M. thanks the NSF for support as an NSF Graduate Research Fellow. The EPR spectroscopy work was supported by the Department of Energy, Office of Basic Energy Sciences, Division of Chemical Sciences grant DE-FG02-05ER15646 (G.B. and G.W.B.). N.H. is a Camille and Henry-Dreyfus Foundation Teacher Scholar.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acscatal.8b00546.
Full characterization data, experimental procedures, and details about EPR simulations (PDF)
X-ray crystallographic data (CIF)
ORCID
Gary W. Brudvig: 0000-0002-7040-1892
Nilay Hazari: 0000-0001-8337-198X
Notes
The authors declare no competing financial interest.
References
- 1.(a) Chirik P, Morris R. Getting Down to Earth: The Renaissance of Catalysis with Abundant Metals. Acc Chem Res. 2015;48:2495–2495. doi: 10.1021/acs.accounts.5b00385. [DOI] [PubMed] [Google Scholar]; (b) Chirik PJ, Gunnoe TB. A Meeting of Metals—A Joint Virtual Issue between Organometallics and ACS Catalysis on First-Row Transition Metal Complexes. ACS Catal. 2015;5:5584–5585. [Google Scholar]
- 2.(a) Rosen BM, Quasdorf KW, Wilson DA, Zhang N, Resmerita A-M, Garg NK, Percec V. Nickel-Catalyzed Cross-Couplings Involving Carbon–Oxygen Bonds. Chem Rev. 2011;111:1346–1416. doi: 10.1021/cr100259t. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Han FS. Transition-Metal-Catalyzed Suzuki–Miyaura Cross-Coupling Reactions: A Remarkable Advance from Palladium to Nickel Catalysts. Chem Soc Rev. 2013;42:5270–5298. doi: 10.1039/c3cs35521g. [DOI] [PubMed] [Google Scholar]; (c) Tasker SZ, Standley EA, Jamison TF. Recent Advances in Homogeneous Nickel Catalysis. Nature. 2014;509:299–309. doi: 10.1038/nature13274. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Ananikov VP. Nickel: The “Spirited Horse” of Transition Metal Catalysis. ACS Catal. 2015;5:1964–1971. [Google Scholar]; (e) Hazari N, Melvin PR, Mohadjer Beromi M. Well-Defined Nickel and Palladium Precatalysts for Cross-Coupling. Nat Rev Chem. 2017:1,0025. doi: 10.1038/s41570-017-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.For reviews about Ni(I) complexes see:; (a) Nag K, Chakravorty A. Monovalent, Trivalent and Tetravalent Nickel. Coord Chem Rev. 1980;33:87–147. [Google Scholar]; (b) Kieber-Emmons MT, Riordan CG. Dioxygen Activation at Monovalent Nickel. Acc Chem Res. 2007;40:618–625. doi: 10.1021/ar700043n. [DOI] [PubMed] [Google Scholar]; (c) Yao S, Driess M. Lessons from Isolable Nickel(I) Precursor Complexes for Small Molecule Activation. Acc Chem Res. 2012;45:276–287. doi: 10.1021/ar200156r. [DOI] [PubMed] [Google Scholar]; (d) Zimmermann P, Limberg C. Activation of Small Molecules at Nickel(I) Moieties. J Am Chem Soc. 2017;139:4233–4242. doi: 10.1021/jacs.6b12434. [DOI] [PubMed] [Google Scholar]; (e) Powers IG, Uyeda C. Metal-Metal Bonds in Catalysis. ACS Catal. 2017;7:936–958. [Google Scholar]; (f) Lin CY, Power PP. Complexes of Ni(I): A ″Rare″ Oxidation State of Growing Importance. Chem Soc Rev. 2017;46:5347–5399. doi: 10.1039/c7cs00216e. [DOI] [PubMed] [Google Scholar]
- 4.(a) Noble A, McCarver SJ, MacMillan DWC. Merging Photoredox and Nickel Catalysis: Decarboxylative Cross-Coupling of Carboxylic Acids with Vinyl Halides. J Am Chem Soc. 2015;137:624–627. doi: 10.1021/ja511913h. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Tellis JC, Kelly CB, Primer DN, Jouffroy M, Patel NR, Molander GA. Single-Electron Transmetalation via Photoredox/Nickel Dual Catalysis: Unlocking a New Paradigm for sp3–sp2 Cross-Coupling. Acc Chem Res. 2016;49:1429–1439. doi: 10.1021/acs.accounts.6b00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.(a) Netherton MR, Fu GC. Nickel-Catalyzed Cross-Couplings of Unactivated Alkyl Halides and Pseudohalides with Organometallic Compounds. Adv Synth Catal. 2004;346:1525–1532. [Google Scholar]; (b) Jones GD, McFarland C, Anderson TJ, Vicic DA. Analysis of Key Steps in the Catalytic Cross-Coupling of Alkyl Electrophiles Under Negishi-Like Conditions. Chem Commun. 2005:4211–4213. doi: 10.1039/b504996b. [DOI] [PubMed] [Google Scholar]; (c) Hu X. Nickel-Catalyzed Cross-Coupling of Non-Activated Alkyl Halides: A Mechanistic Perspective. Chem Sci. 2011;2:1867–1886. [Google Scholar]
- 6.(a) Everson DA, Weix DJ. Cross-Electrophile Coupling: Principles of Reactivity and Selectivity. J Org Chem. 2014;79:4793–4798. doi: 10.1021/jo500507s. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Weix DJ. Methods and Mechanisms for Cross-Electrophile Coupling of Csp2 Halides with Alkyl Electrophiles. Acc Chem Res. 2015;48:1767–1775. doi: 10.1021/acs.accounts.5b00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mohadjer Beromi M, Nova A, Balcells D, Brasacchio AM, Brudvig GW, Guard LM, Hazari N, Vinyard DJ. Mechanistic Study of an Improved Ni Precatalyst for Suzuki–Miyaura Reactions of Aryl Sulfamates: Understanding the Role of Ni(I) Species. J Am Chem Soc. 2017;139:922–936. doi: 10.1021/jacs.6b11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ge S, Green RA, Hartwig JF. Controlling First-Row Catalysts: Amination of Aryl and Heteroaryl Chlorides and Bromides with Primary Aliphatic Amines Catalyzed by a BINAP-Ligated Single-Component Ni(0) Complex. J Am Chem Soc. 2014;136:1617–1627. doi: 10.1021/ja411911s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.(a) Yin G, Kalvet I, Englert U, Schoenebeck F. Fundamental Studies and Development of Nickel-Catalyzed Trifluoromethylthiolation of Aryl Chlorides: Active Catalytic Species and Key Roles of Ligand and Traceless MeCN Additive Revealed. J Am Chem Soc. 2015;137:4164–4172. doi: 10.1021/jacs.5b00538. [DOI] [PubMed] [Google Scholar]; (b) Kalvet I, Guo Q, Tizzard GJ, Schoenebeck F. When Weaker Can Be Tougher: The Role of Oxidation State (I) in P- vs N-Ligand-Derived Ni-Catalyzed Trifluoromethylthiolation of Aryl Halides. ACS Catal. 2017;7:2126–2132. doi: 10.1021/acscatal.6b03344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.For examples where Ni(I) complexes are proposed in cross-coupling and related reactions with sp2 hybridized substrates, see references 4–6 and the following:; (a) Anderson TJ, Jones GD, Vicic DA. Evidence for a Ni(I) Active Species in the Catalytic Cross-Coupling of Alkyl Electrophiles. J Am Chem Soc. 2004;126:8100–8101. doi: 10.1021/ja0483903. [DOI] [PubMed] [Google Scholar]; (b) Jones GD, Martin JL, McFarland C, Allen OR, Hall RE, Haley AD, Brandon RJ, Konovalova T, Desrochers PJ, Pulay P, Vicic DA. Ligand Redox Effects in the Synthesis, Electronic Structure, and Reactivity of an Alkyl–Alkyl Cross-Coupling Catalyst. J Am Chem Soc. 2006;128:13175–13183. doi: 10.1021/ja063334i. [DOI] [PubMed] [Google Scholar]; (c) Phapale VB, Buñuel E, García-Iglesias M, Cardenas DJ. Ni-Catalyzed Cascade Formation of C(sp3)-C(sp3) Bonds by Cyclization and Cross-Coupling Reactions of Iodoalkanes with Alkyl Zinc Halides. Angew Chem, Int Ed. 2007;46:8790–8795. doi: 10.1002/anie.200702528. [DOI] [PubMed] [Google Scholar]; (d) Breitenfeld J, Ruiz J, Wodrich MD, Hu X. Bimetallic Oxidative Addition Involving Radical Intermediates in Nickel-Catalyzed Alkyl–Alkyl Kumada Coupling Reactions. J Am Chem Soc. 2013;135:12004–12012. doi: 10.1021/ja4051923. [DOI] [PubMed] [Google Scholar]; (e) Cherney AH, Kadunce NT, Reisman SE. Catalytic Asymmetric Reductive Acyl Cross-Coupling: Synthesis of Enantioenriched Acyclic α,α-Disubstituted Ketones. J Am Chem Soc. 2013;135:7442–7445. doi: 10.1021/ja402922w. [DOI] [PubMed] [Google Scholar]; (f) León T, Correa A, Martin R. Ni-Catalyzed Direct Carboxylation of Benzyl Halides with CO2. J Am Chem Soc. 2013;135:1221–1224. doi: 10.1021/ja311045f. [DOI] [PubMed] [Google Scholar]; (g) Biswas S, Weix DJ. Mechanism and Selectivity in Nickel-Catalyzed Cross-Electrophile Coupling of Aryl Halides with Alkyl Halides. J Am Chem Soc. 2013;135:16192–16197. doi: 10.1021/ja407589e. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Breitenfeld J, Wodrich MD, Hu X. Bimetallic Oxidative Addition in Nickel-Catalyzed Alkyl–Aryl Kumada Coupling Reactions. Organometallics. 2014;33:5708–5715. doi: 10.1021/ja4051923. [DOI] [PubMed] [Google Scholar]; (i) Schley ND, Fu GC. Nickel-Catalyzed Negishi Arylations of Propargylic Bromides: A Mechanistic Investigation. J Am Chem Soc. 2014;136:16588–16593. doi: 10.1021/ja508718m. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Sayyed FB, Sakaki S. The Crucial Roles of MgCl2 as a Non-Innocent Additive in the Ni-Catalyzed Carboxylation of Benzyl Halide with CO2. Chem Commun. 2014;50:13026–13029. doi: 10.1039/c4cc04962d. [DOI] [PubMed] [Google Scholar]; (k) Börjesson M, Moragas T, Martin R. Ni-Catalyzed Carboxylation of Unactivated Alkyl Chlorides with CO2. J Am Chem Soc. 2016;138:7504–7507. doi: 10.1021/jacs.6b04088. [DOI] [PubMed] [Google Scholar]
- 11.For examples where Ni(I) complexes are proposed in cross-coupling and related reactions with sp3 hybridized substrates, see references 4–6 and the following:; (a) Gao CY, Cao X, Yang LM. Nickel-Catalyzed Cross-Coupling of Diarylamines with Haloarenes. Org Biomol Chem. 2009;7:3922–3925. doi: 10.1039/b911286c. [DOI] [PubMed] [Google Scholar]; (b) Velian A, Lin S, Miller AJM, Day MW, Agapie T. Synthesis and C–C Coupling Reactivity of a Dinuclear Ni(I)–Ni(I) Complex Supported by a Terphenyl Diphosphine. J Am Chem Soc. 2010;132:6296–6297. doi: 10.1021/ja101699a. [DOI] [PubMed] [Google Scholar]; (c) Miyazaki S, Koga Y, Matsumoto T, Matsubara K. A New Aspect of Nickel-Catalyzed Grignard Cross-Coupling Reactions: Selective Synthesis, Structure, and Catalytic Behavior of a T-Shape Three-Coordinate Nickel(I) Chloride Bearing a Bulky NHC Ligand. Chem Commun. 2010;46:1932–1934. doi: 10.1039/b924716e. [DOI] [PubMed] [Google Scholar]; (d) Zhang K, Conda-Sheridan M, Cooke SR, Louie J. N-Heterocyclic Carbene Bound Nickel(I) Complexes and Their Roles in Catalysis. Organometallics. 2011;30:2546–2552. doi: 10.1021/om200090d. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Nagao S, Matsumoto T, Koga Y, Matsubara K. Monovalent Nickel Complex Bearing a Bulky N-Heterocyclic Carbene Catalyzes Buchwald–Hartwig Amination of Aryl Halides under Mild Conditions. Chem Lett. 2011;40:1036–1038. [Google Scholar]; (f) Fujihara T, Nogi K, Xu T, Terao J, Tsuji Y. Nickel-Catalyzed Carboxylation of Aryl and Vinyl Chlorides Employing Carbon Dioxide. J Am Chem Soc. 2012;134:9106–9109. doi: 10.1021/ja303514b. [DOI] [PubMed] [Google Scholar]; (g) Sayyed FB, Tsuji Y, Sakaki S. The Crucial Role of a Ni(I) Intermediate in Ni-Catalyzed Carboxylation of Aryl Chloride with CO2: A Theoretical Study. Chem Commun. 2013;49:10715–10717. doi: 10.1039/c3cc45836a. [DOI] [PubMed] [Google Scholar]; (h) Cornella J, Gómez-Bengoa E, Martin R. Combined Experimental and Theoretical Study on the Reductive Cleavage of Inert C–O Bonds with Silanes: Ruling out a Classical Ni(0)/Ni(II) Catalytic Couple and Evidence for Ni(I) Intermediates. J Am Chem Soc. 2013;135:1997–2009. doi: 10.1021/ja311940s. [DOI] [PubMed] [Google Scholar]; (i) Page MJ, Lu WY, Poulten RC, Carter E, Algarra AG, Kariuki BM, Macgregor SA, Mahon MF, Cavell KJ, Murphy DM, Whittlesey MK. Three-Coordinate Nickel(I) Complexes Stabilised by Six-, Seven- and Eight-Membered Ring N-Heterocyclic Carbenes: Synthesis, EPR/DFT Studies and Catalytic Activity. Chem – Eur J. 2013;19:2158–2167. doi: 10.1002/chem.201202950. [DOI] [PubMed] [Google Scholar]; (j) Correa A, León T, Martin R. Ni-Catalyzed Carboxylation of C(sp2)– and C(sp3)–O Bonds with CO2. J Am Chem Soc. 2014;136:1062–1069. doi: 10.1021/ja410883p. [DOI] [PubMed] [Google Scholar]; (k) Wu J, Nova A, Balcells D, Brudvig GW, Dai W, Guard LM, Hazari N, Lin PH, Pokhrel R, Takase MK. Nickel(I) Monomers and Dimers with Cyclopentadienyl and Indenyl Ligands. Chem – Eur J. 2014;20:5327–5337. doi: 10.1002/chem.201305021. [DOI] [PubMed] [Google Scholar]; (l) Guard LM, Mohadjer Beromi M, Brudvig GW, Hazari N, Vinyard DJ. Comparison of dppf-Supported Ni Precatalysts for the Suzuki–Miyaura Reaction: The Observation and Activity of Ni(I) Angew Chem, Int Ed. 2015;54:13352–13356. doi: 10.1002/anie.201505699. [DOI] [PubMed] [Google Scholar]; (m) Cornella J, Edwards JT, Qin T, Kawamura S, Wang J, Pan CM, Gianatassio R, Schmidt M, Eastgate MD, Baran PS. Practical Ni-Catalyzed Aryl–Alkyl Cross-Coupling of Secondary Redox-Active Esters. J Am Chem Soc. 2016;138:2174–2177. doi: 10.1021/jacs.6b00250. [DOI] [PMC free article] [PubMed] [Google Scholar]; (n) Matsubara K, Fukahori Y, Inatomi T, Tazaki S, Yamada Y, Koga Y, Kanegawa S, Nakamura T. Monomeric Three-Coordinate N-Heterocyclic Carbene Nickel(I) Complexes: Synthesis, Structures, and Catalytic Applications in Cross-Coupling Reactions. Organometallics. 2016;35:3281–3287. [Google Scholar]; (o) Manzoor A, Wienefeld P, Baird MC, Budzelaar PHM. Catalysis of Cross-Coupling and Homocoupling Reactions of Aryl Halides Utilizing Ni(0), Ni(I), and Ni(II) Precursors. Organometallics. 2017;36:3508–3519. [Google Scholar]; (p) Meng QY, Wang S, König B. Carboxylation of Aromatic and Aliphatic Bromides and Triflates with CO2 by Dual Visible-Light–Nickel Catalysis. Angew Chem, Int Ed. 2017;56:13426. doi: 10.1002/anie.201706724. [DOI] [PubMed] [Google Scholar]
- 12.For examples of mechanistic studies on elementary reactions relevant to cross-coupling and related reactions where Ni(I) complexes are involved, see the following:; (a) Tsou TT, Kochi JK. Mechanism of Oxidative Addition. Reaction of Nickel(0) Complexes with Aromatic Halides. J Am Chem Soc. 1979;101:6319–6332. [Google Scholar]; (b) Rettenmeier CA, Wenz J, Wadepohl H, Gade LH. Activation of Aryl Halides by Nickel(I) Pincer Complexes: Reaction Pathways of Stoichiometric and Catalytic Dehalogenations. Inorg Chem. 2016;55:8214–8224. doi: 10.1021/acs.inorgchem.6b01448. [DOI] [PubMed] [Google Scholar]; (c) Yoo C, Ajitha MJ, Jung Y, Lee Y. Mechanistic Study on C-C Bond Formation of a Nickel(I) Monocarbonyl Species with Alkyl Iodides: Experimental and Computational Investigations. Organometallics. 2015;34:4305–4311. [Google Scholar]; (d) Bajo S, Laidlaw G, Kennedy AR, Sproules S, Nelson DJ. Oxidative Addition of Aryl Electrophiles to a Prototypical Nickel(0) Complex: Mechanism and Structure/Reactivity Relationships. Organometallics. 2017;36:1662–1672. [Google Scholar]; (e) Funes-Ardoiz I, Nelson DJ, Maseras F. Halide Abstraction Competes with Oxidative Addition in the Reactions of Aryl Halides with [Ni(PMenPh(3–n))4] Chem – Eur J. 2017;23:16728–16733. doi: 10.1002/chem.201702331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.For other examples of Ni(I) species, see the following:; (a) Eaborn C, Hill MS, Hitchcock PB, Smith JD. The First Structurally Characterised σ-bonded Organonickel(I) Compound. Chem Commun. 2000:691–692. [Google Scholar]; (b) Mindiola DJ, Hillhouse GL. Terminal Amido and Imido Complexes of Three-Coordinate Nickel. J Am Chem Soc. 2001;123:4623–4624. doi: 10.1021/ja010358a. [DOI] [PubMed] [Google Scholar]; (c) Kitiachvili KD, Mindiola DJ, Hillhouse GL. Preparation of Stable Alkyl Complexes of Ni(I) and Their One-Electron Oxidation to Ni(II) Complex Cations. J Am Chem Soc. 2004;126:10554–10555. doi: 10.1021/ja047052z. [DOI] [PubMed] [Google Scholar]; (d) Bai G, Wei P, Stephan DW. A β-Diketiminato–Nickel(II) Synthon for Nickel(I) Complexes. Organometallics. 2005;24:901–5908. [Google Scholar]; (e) Laskowski CA, Hillhouse GL. Two-Coordinate d9 Complexes. Synthesis and Oxidation of NHC Nickel(I) Amides. J Am Chem Soc. 2008;130:13846–13847. doi: 10.1021/ja805804s. [DOI] [PubMed] [Google Scholar]; (f) Hamacher C, Hurkes N, Kaiser A, Klein A, Schüren A. Electrochemistry and Spectroscopy of Organometallic Terpyridine Nickel Complexes. Inorg Chem. 2009;48:9947–9951. doi: 10.1021/ic900753r. [DOI] [PubMed] [Google Scholar]; (g) Anderson JS, Iluc VM, Hillhouse GL. Reactions of CO2 and CS2 with 1,2-Bis(di-tert-butylphosphino)ethane Complexes of Nickel(0) and Nickel(I) Inorg Chem. 2010;49:10203–10207. doi: 10.1021/ic101652e. [DOI] [PubMed] [Google Scholar]; (h) Davies CJE, Page MJ, Ellul CE, Mahon MF, Whittlesey MK. Ni(I) and Ni(II) Ring-Expanded N-Heterocyclic Carbene Complexes: C-H Activation, Indole Elimination and Catalytic Hydrodehalogenation. Chem Commun. 2010;46:5151–5153. doi: 10.1039/c0cc01335h. [DOI] [PubMed] [Google Scholar]; (i) Beck R, Shoshani M, Krasinkiewicz J, Hatnean JA, Johnson SA. Synthesis and Chemistry of Bis(triisopropylphosphine)-nickel(I) and Nickel(0) Precursors. Dalton Trans. 2013;42:1461–1475. doi: 10.1039/c2dt32008h. [DOI] [PubMed] [Google Scholar]; (j) Lipschutz MI, Tilley TD. Carbon–Carbon Cross-Coupling Reactions Catalyzed by a Two-Coordinate Nickel(II)–Bis(amido) Complex via Observable Ni(I), Ni(II), and Ni(III) Intermediates. Angew Chem, Int Ed. 2014;53:7290–7294. doi: 10.1002/anie.201404577. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Lipschutz MI, Tilley TD. Useful Method for the Preparation of Low-Coordinate Nickel(I) Complexes via Transformations of the Ni(I) Bis(amido) Complex K{Ni[N(SiMe3)(2,6-iPr2-C6H3)]2} Organometallics. 2014;33:5566–5570. doi: 10.1021/om500849u. [DOI] [PMC free article] [PubMed] [Google Scholar]; (l) Rettenmeier C, Wadepohl H, Gade LH. Stereoselective Hydrodehalogenation via a Radical-Based Mechanism Involving T-Shaped Chiral Nickel(I) Pincer Complexes. Chem – Eur J. 2014;20:9657–9665. doi: 10.1002/chem.201403243. [DOI] [PubMed] [Google Scholar]; (m) Yoo C, Oh S, Kim J, Lee Y. Transmethylation of a Four-Coordinate Nickel(I) Monocarbonyl Species with Methyl Iodide. Chem Sci. 2014;5:3853–3858. [Google Scholar]; (n) Schwab MM, Himmel D, Kacprzak S, Radtke V, Kratzert D, Weis P, Wernet M, Peter A, Yassine Z, Schmitz D, Scheidt EW, Scherer W, Weber S, Feuerstein W, Breher F, Higelin A, Krossing I. Synthesis, Characterisation and Reactions of Truly Cationic NiI–Phosphine Complexes. Chem – Eur J. 2018;24:918–927. doi: 10.1002/chem.201704436. [DOI] [PubMed] [Google Scholar]
- 14.(a) Laskowski CA, Bungum DJ, Baldwin SM, Del Ciello SA, Iluc VM, Hillhouse GL. Synthesis and Reactivity of Two-Coordinate Ni(I) Alkyl and Aryl Complexes. J Am Chem Soc. 2013;135:18272–18275. doi: 10.1021/ja4095236. [DOI] [PubMed] [Google Scholar]; (b) Hatnean JA, Shoshani M, Johnson SA. Mechanistic Insight into Carbon–Fluorine Cleavage with a (iPr3P)2Ni Source: Characterization of (iPr3P)2NiC6F5 as a Significant Ni(I) Byproduct in the Activation of C6F6. Inorg Chim Acta. 2014;422:86–94. [Google Scholar]; (c) In reference 10i, Schley and Fu describe the complex ((−)-iPr-pybox)NiI(Ph), which is formally a Ni(I) species. However, EPR spectroscopy indicates that it is a Ni(II) complex with a ligand-centered radical on the pybox. The complexes described in reference 13f are also Ni(II) complexes with ligand-centered radicals.
- 15.For example, the biaryl products could arise if the Ni(I) aryl species underwent disproportionation to generate (dppf)NiII(Ar)2 and a zerovalent Ni species; see for example:; Bajo S, Laidlaw G, Kennedy AR, Sproules S, Nelson DJ. Organometallics. 2017;36:1662. [Google Scholar]; Yamamoto T, Wakabayashi S, Osakada K. J Organomet Chem. 1992;428:223. [Google Scholar]; followed by reductive elimination from (dppf) NiII(Ar)2 to generate a biaryl and a Ni(0) product. Alternatively, the same products could be generated through direct bimetallic reductive elimination from the Ni(I) aryl species; see for example:; Xu H, Diccianni JB, Katigbak J, Hu C, Zhang Y, Diao T. J Am Chem Soc. 2016;138:4779. doi: 10.1021/jacs.6b00016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


