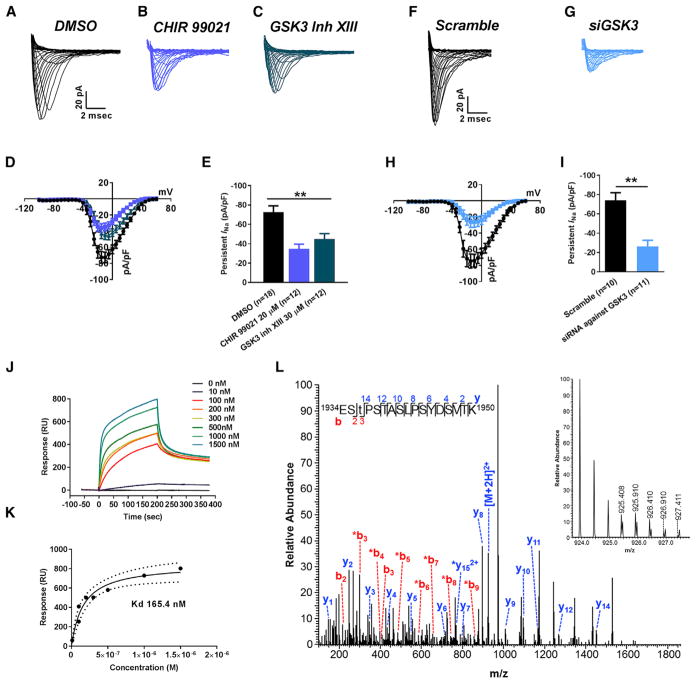
Figure 5. In Vitro Studies of Functional Interaction between Nav1.6-Encoded Currents and GSK3.
(A–C) Representative traces of transient INa recorded from Nav1.6-HEK293 cells treated with DMSO (A), CHIR 99021 (B), and GSK3 inhibitor XIII (C).
(D and E) Nav1.6 current-voltage relationship of DMSO or CHIR 99021 or GSK3 inhibitor XIII treatment (D), and peak current density at −10 mV voltage step (E).
(F and G) Representative traces of transient INa recorded from Nav1.6-HEK293 cells treated with siScramble (F) and siGSK3 (G).
(H and I) Nav1.6 current-voltage relationship of siScramble versus siGSK3 (H), and peak current density at −10 mV voltage step (I).
(J) Representative SPR sensorgram of GSK3β binding to Nav1.6 C-tail.
(K) SPR fitting curve of GSK3β binding with Nav1.6 C-tail.
(L) Higher energy collisional dissociation (HCD) fragmentation spectrum of the phosphopeptide EStPSTASLPSYDSVTK, encompassing residues 1934–1950 of the Nav1.6 C terminus. The presence of non-phosphorylated b2 (theoretical m/z of 217.08, observed m/z of 217.08) and y14 (theoretical m/z of 1,452.72, observed m/z of 1,452.72) ions along with phosphorylated b3 (theoretical m/z of 398.10, observed m/z of 398.10) confirms T1936 as the site of phosphorylation. The parent ion was fragmented with a co-eluting peptide within the ±2 Da window (inset) resulting in the mixed spectrum shown here. t, phosphothreonine; *, fragment with loss of phosphoric acid). Data are represented as mean ± SEM. *p < 0.05, **p < 0.01; Student’s t test.