ABSTRACT
Use of monoclonal antibodies is emerging as a highly promising and fast-developing scenario for innovative treatment of viral, autoimmune and tumour diseases. The search for diagnostic and therapeutic antibodies currently depends on in vitro screening approaches, such as phage and yeast display technologies. Antibody production still represents a critical step for preclinical and clinical evaluations. Accordingly, improving production of monoclonal antibodies represents an opportunity, to facilitate downstream target validations. SINEUP RNAs are long non-coding transcripts, possessing the ability to enhance translation of selected mRNAs. We applied SINEUP technology to semi-stable production of monoclonal antibodies in HEK293E cells, which allows for episomal propagation of the expression vectors encoding the heavy and light chains of IgGs. Co-expression of SINEUP RNA with mRNAs encoding heavy and light chains of IgG4s was able to increase the production of different anti-CLDN1 antibodies up to three-fold. Improved production of monoclonal antibodies was achieved both in transiently transfected HEK293E cells and in cellular clones with stable expression of the SINEUP. Compared to antibody preparations obtained under standard conditions, the anti-CLDN1 IgG4s produced in the presence of the SINEUP transcript showed unaltered post-translational modifications, and retained the ability to recognize their target. We thus propose SINEUP technology as a valuable tool to enhance semi-stable antibody production in human cell lines.
KEYWORDS: SINEUP, lncRNA, monoclonal antibodies, antibody production, IgG4, HEK293E, glycosylation, scFv, CLDN1
Introduction
Monoclonal antibodies (mAbs) represent useful tools in many biological fields, for research, diagnostic and therapeutic approaches for viral infections and cancer diseases. To date, tens of mAbs are well-established therapeutics, and additional clinical trials, especially related to cancer immunotherapy, arise over time, keeping pace with their growing potential. The global market for mAbs reached about USD 80 billion in 2015 and, according to current compound annual growth rate, it is expected to increase to nearly USD 140 billion by 2024. 1
The hybridoma approach, pioneered by Köhler and Milstein in 1975, was the most used technology to isolate and produce antibodies for many years. 2 More recently, in vitro production of mAbs revolutionized the way to isolate and produce mAbs and, to date, these methods represent the default way to perform screenings against new targets. 3 The most powerful way to isolate recombinant mAbs is the application of phage/yeast display of single-chain variable fragment (scFv) libraries. 4 These systems allow, potentially, the identification of specific scFv fusions for any target of interest, from a huge scFv repertoire, through biopanning. Subsequently, both VH and VL fragments are isolated from the scFv sequence and subcloned into dedicated expression vectors for yeast or mammalian cell lines, to reconstitute a fully human antibody. The choice of the vector allows production of any isotype of immunoglobulin for specialized downstream applications.
A wide variety of production systems have been set up, including non-mammalian and mammalian platforms. The first class comprises simple organisms as prokaryotic, yeast, fungi or insect cells whose advantages are low costs and good productivity (in the high mg/L range). 5 Sub-optimal folding and a very different pattern of glycosylation make these systems useful for production of simple non-glycosylated proteins, or for generation of preliminary binding data. Despite relatively high production costs, more than 95% of mAbs approved by the Food and Drug Administration (FDA) are produced in mammalian cell lines, which allow post-translational modifications (PTMs) (i.e., glycosylation) similar to those made in humans. 6 , 7 The mammalian expression platforms include hamster, mice and human cell lines. Among the most used mammalian non-human cell lines are the Chinese hamster ovary (CHO), hamster kidney (BHK), murine NS0, SP2/0 and C127 cells. To date, most of the recombinant proteins, in particular mAbs, are produced in CHO cells, although the PTMs of CHO cells may not be optimal. Indeed, hamster cells produce non-human N-glycosylation named NGNA and α-Gal, and lack human modifications, such as fucosylation and sialylation. 6 , 8 A great debate is still ongoing, since non-human PTMs may affect both efficacy of antibody-dependent cell-mediated cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC), and they could generate immunogenicity against recombinant mAbs. 9 To generate human-compatible mAbs in CHO cells, a great effort is dedicated to isolate variants with an acceptable glycosylation profile.
Recently, biotherapeutics produced in HEK293, HT1080 and PER C6 human cell lines have been approved by FDA or the European Medicines Agency. 7 Although the generation of a stable cell line expressing high levels of mAbs is the final goal for clinical development, it still remains time-consuming and expensive during the development phases of the research, before occurrence of preclinical and clinical studies. Thus, to get cheap, fast and parallel production of many recombinant mAbs, transient or semi-stable cell culturing is much more appropriate. The HEK293 cell line is very useful to this aim because it is highly proliferative, easily grown and transfected with high efficiency. HEK293-EBNA cells, also named HEK293E, are stably transfected with Epstein-Barr virus protein EBNA-1, which allows propagation of vectors containing an origin of replication (ORI) as episome in a semi-stable manner. In addition, HEK293E cells can be cultured in chemically defined media, thus avoiding the risk of contamination by bovine immunoglobulins and pathogens potentially contained in media complemented with fetal bovine serum. 10 Eventually, to scale up the mAb productivity, HEK293E cells, which grows in suspension, can be cultured in fed-batch or in bioreactors. All these features make HEK293E a convenient system to produce mAbs for both basic and advanced research-grade applications, in vitro and in vivo. Since the bottleneck of transient production of mAbs in human cell lines is the productivity, many attempts to improve the moderate yield from tens to hundreds of mg/L have been made. Indeed, to maximize productivity, it is possible to improve cell viability, or enhance the transcription, translation, folding and secretion of mAbs. Since apoptosis is the main cause of cell death during cultivation, different anti-apoptotic engineering approaches have been exploited to select apoptosis-resistant cells by, for instance, overexpression of anti-apoptotic Bcl-2 family proteins, human telomerase reverse transcriptase (hTert), or acting on cell cycle through p53, cyclin E, E2F-1, p21, p27 and p18. 6 , 11 , 12 Other authors have focused on enhancing protein folding or reducing unfolded protein response (UPR), typical conditions of highly producing cell lines. 13 Thanks to these engineering approaches, to date, very good productivity in HEK293 cells, up to about 300 mg/L has been reported. 12 , 13 Despite this considerable productivity, these engineered cell lines remain useful only for research-level characterizations because the non-target-specific boosting of production and the enhanced tumorigenic potential of the modified cell lines makes their approval by regulatory agencies questionable.
A new class of natural and synthetic long non-coding RNAs has been recently described, that increases the translation of specific mRNA targets. 14 SINEUP RNAs work by a 5′ head-to-head divergent perfect complementarity with the target mRNA. Namely, the region encompassing the 5′UTR and the first AUG codon of the target mRNA is bound by the 5′ end of the long non-coding RNA. The activity of the SINEUP transcripts depends on two main domains: a target-specific binding domain and a universal effector domain containing a modular structure with inverted SINE elements, belonging to the B2 subclass (SINEB2 element) and partial Alu element. 15 Moreover, it has been reported that SINEUPs work in several mammalian cells, including mouse, hamster, monkey and human cell lines. To date, a range of 1.5 to 3 times increases in recombinant protein production by SINEUP have been reported for both cytoplasmic and secreted proteins, according to cell- and target-specificity. 16 A first attempt to increase mAb production through SINEUP reported by Yao et al. shows a potential usefulness of the system even if with a seemingly limited efficacy, with 50% increases in production of antibodies. 17 In this paper, we implemented SINEUP technology to boost semi-stable production of mAbs in HEK293E cells, demonstrating its validity, in terms of improved antibody production, without losses in post-translation modifications and binding ability of the SINEUP-produced antibodies.
Results
Construction of expression vectors for SINEUP-mediated expression of monoclonal antibodies
To generate a versatile tool for production of mAbs that could be useful for research and advanced biomedical applications, we addressed the possibility of boosting the production of IgG4 mAbs in HEK293E cells, co-transfected with heavy and light chain expression vectors, in the presence of a suitable SINEUP construct. The optimization of SINEUP sequences by Yi Yao et al. suggested that the classical binding domain (-40+32) of SINEUP is highly efficient in enhancing target production. 17 Accordingly, we designed a specific canonical binding domain targeting the -40 to +32 sequence, encompassing the first ATG codon within the IgG4 heavy chain vector pEUVH8.2, and cloned it upstream of the effector domain SINEB2. The full-length SINEUP construct (SINEUP-40+32) was indeed generated into the pcDNA3.1Hygro vector, under the control of the CMV promoter. This vector should improve the production of the antibodies heavy chains expressed from pEUVH8.2, but it would be inefficient for the light chain-encoding vector pEUVL4.2, due to slightly divergent sequences in the 5′ regions, preventing full pairing with the SINEUP-40+32 transcript. Supplementary Figures S1A and S1B describe the strategy used for generation of the pEUVL4.2_SINEUP_Adapted vector (pEUVL4.2_SA) and its validation, respectively. The reference antibody selected for our study was the anti-CLDN1 mAb B9x. 18
Next, we verified the performance of the SINEUP-40+32 construct in the production of B9x antibody in transiently transfected HEK293E cells. To this aim, standard quantities of pEUVH8.2_B9x and pEUVL4.2_SA_B9x were co-transfected in the presence of the SINEUP-40+32 construct, or with its empty vector, pcDNA3.1Hygro. We used a molar ratio of 1:6 (target vectors to SINEUP construct). A constant amount of reporter pGLuc Mini-TK, expressing a secreted Gaussia luciferase, was co-transfected as internal reference for transfection efficiency normalization. Antibody production was assessed 48 h post transfection by sandwich ELISA of cell culture supernatants. As shown in Fig. 1A, a sharp 4-fold increase of B9x mAb production by the SINEUP-40+32 was reached, in comparison to the empty vector. The ELISA assay was specific for fully assembled IgGs, so that these results show that the SINEUP-40+32 RNA actually targets both heavy and light chains, and that the antibodies produced are correctly processed and secreted into functional tetramers.
Figure 1.
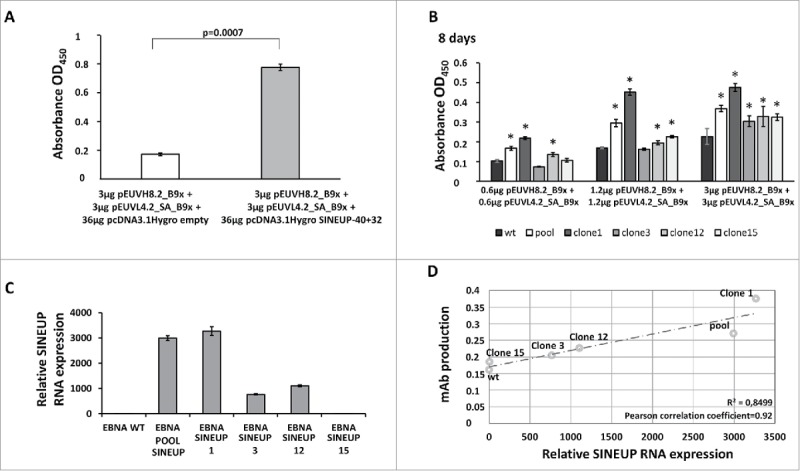
Increased production of anti-CLDN1 B9x IgG4 by transient and stable expression of the SINEUP-40+32 construct>. A. The ELISA assay shows increased production of full human B9x IgG4 in cells co-transfected with the construct expressing the SINEUP-40+32 lncRNA, targeting both heavy- and light-chain mRNAs of the anti-CLDN1 B9x antibody. B. The ELISA assay, revealing full human IgGs, shows the antibodies secreted by HEK293E (wt) and HEK293E_SINEUP-40+32 cells stably expressing the lncRNA (pool or isolated clones 1, 3, 12, 15) after 8 days following transfection with pEUVH8.2_B9x and pEUVL4.2_SA_B9x vectors. The asterisks indicate p values < 0.05 (compared to wt cells). C. The panel shows the levels of SINEUP-40+32 lncRNA transcripts in HEK293E (wt) cells and in HEK293E_SINEUP-40+32 clones. D. The chart reports the results of a correlative analysis of anti-CLDN1 B9x mAb production (y axis) against relative levels of SINEUP-40+32 lncRNA transcripts (x axis). B9x expression data used for comparisons were averaged from the whole set of results shown in the panel B of the Figure.
Generation of stable cell lines expressing the SINEUP-40+32 lncRNA
The final goal of our study was to generate a system to boost production of mAbs, overcoming the limitations linked to co-transfection of several vectors. Therefore, we focused on developing a cell line stably expressing sufficiently high levels of the SINEUP-40+32. Moreover, this approach would have taken full advantage of the production system of the HEK293E cell line, which permits propagation, in semi-stable manner and over the production timespan, of the episomal pEU vectors expressing heavy and light chains. On the contrary, the transiently transfected SINEUP-40+32 would lose the effect of boosting because of the dilution of the vector during cell divisions. Hence, thanks to the hygromycin selection marker within the SINEUP-40+32 construct, we selected isolated clones, as well as a pool of antibiotic-resistant cells, with stable expression of the lncRNA. Next, we co-transfected the selected clones with different amounts of pEUVH8.2_B9x and pEUVL4.2_SA_B9x constructs, to evaluate their productivity rates. As shown in Fig. 1B, we monitored the levels of secreted mAbs after 8 days following transfection. In comparison to wild-type HEK293E cells, we identified the clone 1 and the pool as the most effective mAb producer cells; clones 3 and 12 acted as moderate producers, while clone 15 behaved similarly to the wild-type HEK293E cell line.
To validate that the high yield of recombinant mAb was correlated to the expression of the SINEUP-40+32 lncRNA transcripts, we analyzed its levels by quantitative RT-PCR in all selected clones (Fig. 1C). The direct effect of SINEUP-40+32 was demonstrated by a correlation index of 0.92 between the levels of mAb production and lncRNA expression (Fig. 1D). We selected the clone 1 as the best cell line, and we named it HEK293EBNA_SINEUP_1 (abbreviated as HEK293ES_1). Thus, we selected HEK293ES_1 cells, to verify the universality of the boosting effect; accordingly, additional anti-Claudin-1 IgG4-based antibodies, 18 D10x and H9C1, were produced. As shown in Fig. 2A, the boosting effect by HEK293ES_1 cells expressing the SINEUP-40+32 lncRNA was clearly occurring also for the antibodies D10x and H9C1, with increases in production up to three times. The D10x antibody was produced at the highest levels, approaching 300 mg/L.
Figure 2.
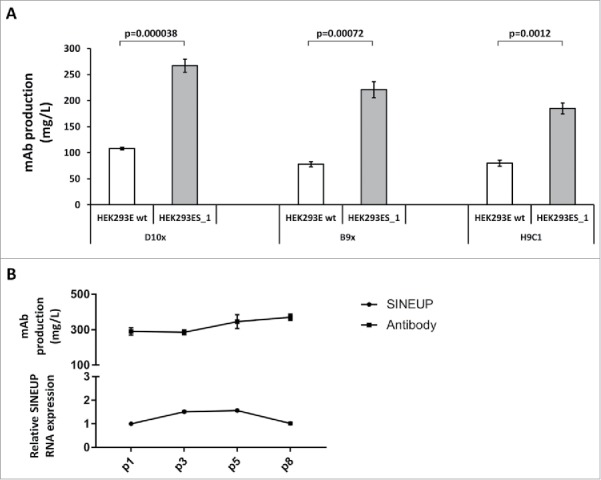
SINEUP-40+32 lncRNA transcripts boost mAb production for a variety of IgG4s and enable stable mAb production yields. A. The chart reports the values of average productions for anti-CLDN1 IgG4 antibodies D10x, B9x and H9C1 in wild-type HEK293E cells, and in the HEK293ES_1, stably expressing the SINEUP-40+32 lncRNA transcript. B. The chart shows the relative levels of the SINEUP-40+32 transcripts (bottom) and the absolute yields of B9x antibody (top) after the indicated, consecutive passages of the HEK293ES_1 cells, stably expressing the lncRNA.
In order to validate the suitability of the HEK293ES_1 cell line for large-scale productions, we verified SINEUP-40+32 lncRNA transcript levels and B9x mAb production over 8 consecutive passages. As shown in Fig. 2B, both SINEUP expression and mAb production were stable over time.
Improvements in production by SINEUP do not alter the PTM pattern and the binding properties of the mAbs
PTMs, especially glycosylations, have a central role in stability and activity of antibodies. Altered glycosylation patterns could indeed be occurring, due to the overproduction of antibodies. Thus, we decided to study potential differences in glycosylation patterns in mAb B9x expressed in HEK293E wild-type cells, or in HEK293ES_1. To this aim, we performed a 2-D gel electrophoresis of B9x antibodies purified from both cell lines. 19 The single antibody preparations were labelled with the fluorescent dyes, Cy3 for HEK293ES_1-derived preparation (Fig. 3A) and Cy5 for wild-type HEK293E cells (Fig. 3B); an equimolar mixture of the two antibody preparations was also labelled with Cy2 for normalization (Fig. 3C), according to a 2 Dimension-Differential In-gel Analysis (2D-DIGE) procedure. As shown in Fig. 3C, multiple spots of 50 (H1 to H7) and 25 kDa (L1 to L2) were identified for heavy and light chains, respectively, in both antibody preparations. The patterns of 2D separations and the relative levels of each spot were similar for both antibody preparations, irrespective of their source, as demonstrated by the merged image in Fig. 3D. The protein spots were finally analyzed within the Differential In-gel Analysis procedure. The results, summarized in Fig. 3E, show that the normalized volume ratios for each protein spot are very close to 1, indicating a similar extent of representation of each spot between the two samples. We also performed characterization of glycoforms by mass spectrometry for both standard and boosted antibody preparations (Supplementary Table 1). B9x mAb produced in HEK293E or in HEK293ES_1 cells showed identical relative representation of both major and minor glycoforms. Taken together, these data demonstrate that, despite the higher levels of mAb production obtained with the HEK293ES_1 cells, no differences are present in the post-translation modification patterns of the two antibody preparations.
Figure 3.
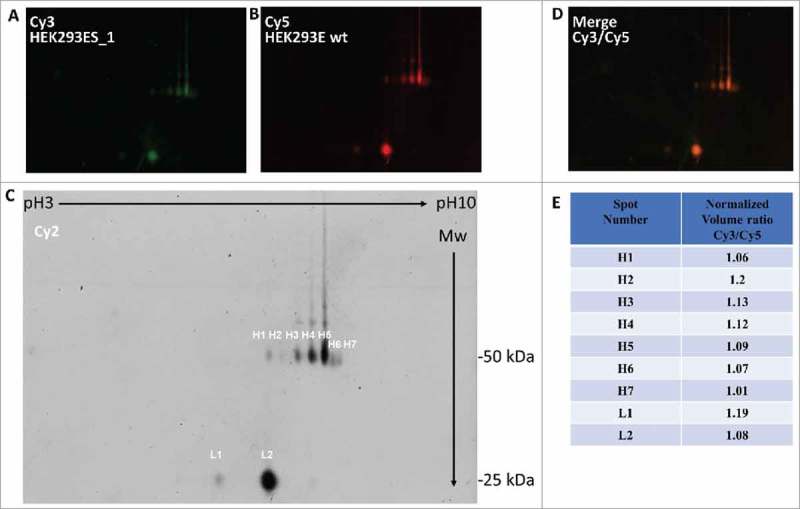
2D-DIGE analysis of antibodies produced by HEK293E and HEK293ES_1 cells. The Figure shows the bi-dimensional separation of labeled B9x preparations from HEK293ES_1 (Cy3, panel A), or from wt HEK293E (Cy5, panel B) cells. The enlarged panel C shows a 1:1 mixture of the two preparations (Cy2-labeled), in which the relevant spots are indicated (H1 to H7 for the heavy chain; L1 to L2 for the light chain). The panel D shows the merged image of panels A and B; the spot volume ratios for Cy3 vs. Cy5 samples, normalized according to the volumes of the Cy2 label, as from a differential in-gel analysis (DIA) software, are indicated in the panel E.
Finally, we compared by fluorescence-activated cell sorting (FACS) analysis the binding ability of mAb B9x produced in HEK293E or in HEK293ES_1 cells. As shown in Fig. 4A, the binding ability assessed on CLDN-1-expressing Huh7.5 cells results in similar binding levels between the two different preparations. Furthermore, we evaluated binding affinities for the B9x mAb produced in HEK293E or in HEK293ES_1 cells by ELISA assays on Huh7.5 cells, over a wide range of antibody concentration. The two curves show that both antibody preparations reach maximal binding with highly similar profiles (Fig. 4B). Taken together, these experiments show that the B9x mAb produced in in HEK293ES_1 cells fully retains binding and affinity properties compared to mAb produced under standard conditions.
Figure 4.
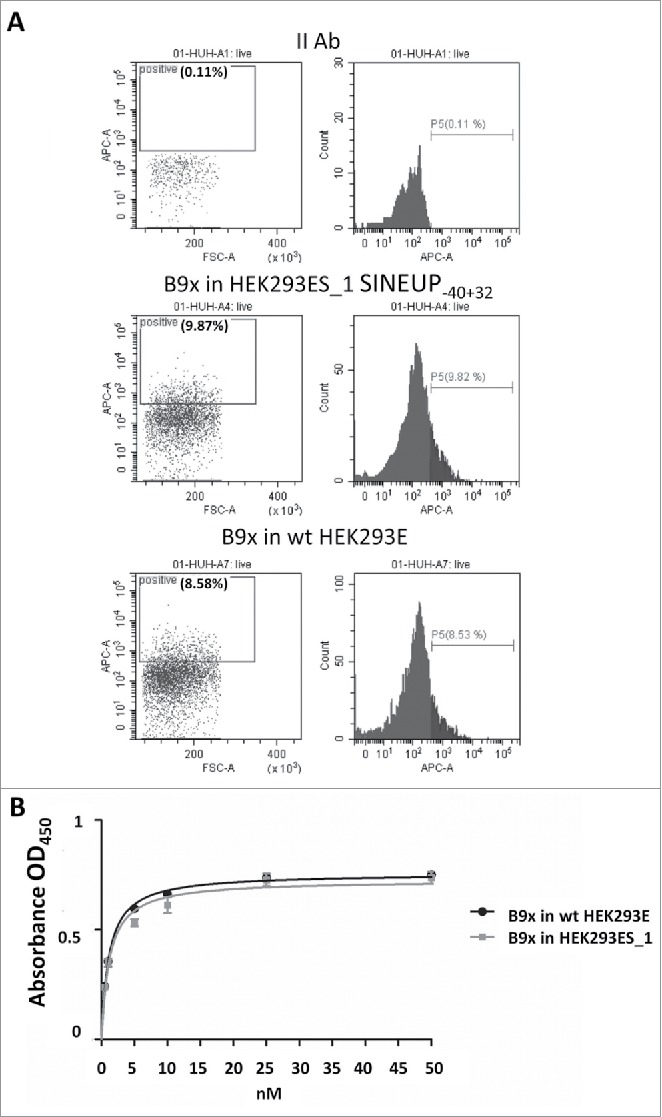
Binding properties of B9x antibodies produced in HEK293E and HEK293ES_1 cells on CLDN1-expressing HuH7 cells.A. The charts report the surface staining of HuH7 cells with the anti-CLDN1 B9x antibody produced in SINEUP-40+32 expressing HEK293ES_1 (center) or in wild-type HEK293E (right) cells. The charts on the left show the control staining with the secondary antibody (II Ab). B. Cell ELISA assay of HuH7 cells with the B9x antibody produced in SINEUP-40+32 expressing HEK293ES_1 or in wild-type HEK293E cells over a wide range of antibody concentrations.
Discussion
Here, we describe a biotechnological approach to boost antibody production for biomedical applications, relying on the SINEUP RNAs, a recently identified class of transcripts belonging to the long non-coding RNA molecules. Since their discovery, SINEUPs have been shown to be widely useful for the production of recombinant proteins, including antibodies. 17 The approach we selected is based on the implementation of SINEUP technology in the well-established semi-stable production system of HEK293-EBNA cells. These cells support expression of proteins from episomal plasmids, such as the pEU vectors used in this study. To validate the potential application of SINEUP to the improved production of mAbs, we undertook an approach based on both transient and stable expression of an optimized SINEUP construct with episomal vectors encoding for the heavy and light chains of IgG4 mAbs targeting CLDN-1, a membrane protein mediating hepatitis C virus (HCV) entry. 18 The improvements in antibody production by the SINEUP construct were effective under both conditions, demonstrating its usefulness, with no cellular toxicity for stable expression of the long non-coding RNA. We developed optimization of IgG4 production within a pipeline of antibody screening and characterization typical of research and development activities, downstream of high-throughput screening of scFv repertoires. 18 , 20 The system can be easily adapted to larger scale production of antibodies, using the HEK293ES_1 cells as recipient line, for applications required within preclinical uses and further.
The antibodies produced by HEK293ES_1 cells expressing the SINEUP-40+32 were undistinguishable from those purified from the classical HEK293E cell line, according to their PTM patterns and binding abilities. Thus, our approach shows that boosting mAb production with SINEUP is compatible with downstream applications of the purified antibodies. Accordingly, we improved the production of CLDN-1 antibodies up to three-fold, without losses in binding activity and in assembly or glycosylation pattern. This allows confirmation of the robustness of the combination of SINEUP technology to semi-stable production of mAbs with episomal vectors in HEK293ES_1 cells. Further studies will be required to evaluate whether the improvements in production in SINEUP-expressing cells is associated with enhanced endoplasmic reticulum stress.
We demonstrated that our system of improved antibody production of mAbs works for the IgG4 class, but there are no predictable limitations for the extension of this approach to additional classes of immunoglobulins, or to secreted proteins. The design of the SINEUP-40+32 encompasses the translation initiation codon AUG of vectors encoding for both the heavy and light chains of IgGs; the binding domain of the SINEUP extends within the leader peptide of both chains, which is removed during the trafficking of the expressed protein within endoplasmic reticulum. This opens the way to more general uses of the HEK293ES_1 cells, i.e., for production of secreted proteins, such as Fc-based fusions of recombinant proteins. Our SINEUP system adds to those already established for optimization of mAb production.11-13 The combination of SINEUP technology with existing and upcoming technologies will be evaluated in the future, to verify whether optimal mAb production can be reached, in terms of even more pronounced yields and unaltered PTMs.
Materials and methods
Cell cultures and manipulation and antibodies production
The HEK293E cell line was purchased (Invitrogen) and cultured in standard conditions using DMEM complemented with 1% penicillin/streptomycin, 2 mM glutamine, 10% fetal bovine serum, 1% non-essential amino acids and 250 µg/ml G418 (all from Gibco, Life Technologies, Inc.) at 37°C, in 5% CO2 humidified atmosphere. For propagation of HEK293 EBNA SINEUP-40+32 cell lines, the DMEM medium was also supplemented with 100 µg/ml Hygromycin B (Invitrogen). Huh7.5 cells were cultured in DMEM containing 1% penicillin/streptomycin, 2 mM glutamine, 10% fetal bovine serum, 1% non-essential amino acids at 37°C, in 5% CO2 humidified atmosphere. Transfections were performed using different amounts (as indicated above) of the recombinant pEU vectors encoding heavy and light chains or SINEUP coding vector. All the transfections were performed with Lipofectamine® Transfection Reagent (Life Technologies, Inc.) using a DNA (µg) to Lipofectamine (µL) ratio of 1:2.5. Six hours after transfection, DMEM medium was replaced with chemically defined CD CHO medium (Gibco, Life Technologies, Inc.). For binding assays and 2D-DIA application, the antibodies secreted in the medium were purified by using HiTrap Protein-A HP (GE Healthcare Life Sciences).
DNA constructs
pEU4.2 vector was modified by using QuikChange XL Site-Directed Mutagenesis Kit (Agilent Technologies, Inc). SINEUP backbone was purchased from Cell Guidance Systems in pUC19 containing the effector domain (ED). The binding domain was designed over the -40 to +32 of target sequences and synthesized as two overlapping oligonucleotides, BD_US and BD_LS (sequences reported below) with XhoI and EcoRV compatible sites, respectively, at their 5′ ends.
BD_US: 5′-TCGAGTACCAAGAAGAGGATGATACAGCTCCATCCCATGGTGGCCACGTGTTCACGACACCTGAAATGGAAGAAAAAAGAT-3′
BD_LS: 5′-ATCTTTTTTCTTCCATTTCAGGTGTCGTGAACACGTGGCCACCATGGGATGGAGCTGTATCATCCTCTTCTTGGTAC-3′
After oligonucleotide annealing, the dsDNA fragment was ligated with Rapid DNA ligation Kit (Roche) into pUC19 vector upstream ED, previously digested by XhoI and EcoRV (New England Biolabs). The whole SINEUP (BD+ED) was then sub-cloned by In-Fusion™ HD cloning kit (Clontech Laboratories, Inc.) into pcDNA3.1Hygro+ previously digested with BamHI and XbaI restriction endonucleases (New England Biolabs).
ELISA assays
To assess the production of fully assembled mAbs, we used Human IgG ELISA Quantification set (Bethyl Laboratories, Inc.). Briefly, anti-Human IgG (100 μl from a 10 µg/ml solution) were coated in NuncTM flat-bottom 96-well plates (Thermo Fisher Scientific) in a solution of 0.05 M NaHCO3 for 24 hours. The blocking of the coated 96-well plates was performed with 0.05% Tween (volume/volume) and 5% bovine serum albumin (BSA) (weight/volume) in phosphate-buffered saline (PBS) for 1 hour at 37°C. After 5 washes with 1x PBS (Lonza Walkersville, Inc.), the conditioned media were added at different concentration (1:1 to 1:1000), diluted in 0.05% Tween and 1% BSA in PBS, and incubated for 2 hours at room temperature by gently shaking. After washes with PBS, the plates were incubated with horseradish peroxidase (HRP)-conjugated goat anti-human IgG-Fc detection antibody, diluted in 0.05% Tween and 1% BSA in PBS for 45′ at RT. Then, plates were washed again and incubated with TMB reagent before quenching with an equal volume of 1 N HCl. Absorbance at 450nm was finally evaluated; statistical significance of differences in mAbs productions were carried out according to Student's t-test.
Cell ELISA assays were performed on CLDN-1-expressing Huh7.5 cells. Cells were plated in round-bottom 96-well plates (3.105 cells/well) and incubated with mAbs, used at increasing concentrations (0.5, 1, 5, 10, 25, 50 nM) in 2.5% Nonfat Dry Milk for 2 hours at room temperature in agitation (150 rpm). Cells were washed three times with PBS and then incubated with HRP-conjugated anti-human IgG goat polyclonal secondary antibody for 1 hour at room temperature. Next, cells were washed three times with PBS, TMB reagent was added before quenching with an equal volume of 1 N HCl. Absorbance was measured at 450nm, using an Envision plate reader (Perkin Elmer). The binding curve values were reported as the mean of three determinations obtained in the experiment.
Luciferase assays
pGLuc Mini-TK 2 vector (New England Biolabs) expressing a secreted Gaussia luciferase was co-transfected with SINEUP and pEU vectors in ratio of 1:10 (µg/µg) pGLuc Mini-TK 2/pEU+pcDNA3.1. The secreted Gaussia luciferase activity was dosed from 20 µL of conditioned media by BioLux® Gaussia Luciferase Assay Kit (New England Biolabs) in black plates.
Quantitative PCR analysis
The lnc SINEUP expression in the isolated clones (pool, 1, 3, 12, 15) was evaluated by using quantitative RT-PCR. Cells were lysed by TriFast (EuroClone) and the total RNA was extracted with phenol/chloroform. 5 µg of RNA were treated with RQ1 RNase-free DNase (Promega) to eliminate residual DNA contaminants. After DNase inactivation, 1 µg of RNA was reverse-transcribed by using ImProm-II Reverse Transcriptase (Promega) in a mix containing 3 mM MgCl2, 0.5mM dNTP and 500 ng random primer examer mixture (Invitrogen). The cDNA was then amplified in a 7500 Real-Time PCR System (Applied Biosystem) using SYBR Green PCR Mastermix (Applied Biosystem). All oligonucleotide primers (listed below) were used to a final concentration of 0.2 µM.
ACTB FOR: 5′-CGTGCTGCTGACCGAGG-3′
ACTB REV: 5′-GAAGGTCTCAAACATGATCTGGGT-3′
SINEUP_FOR: 5′-TCCTGTGCAAGAGCATCCAG-3′
SINEUP_REV: 5′-TCCCTTGCTGTTCGTTCGTT-3′
The relative abundance of target RNAs was evaluated in relation to β-actin (ACTB) transcript by ΔΔCt method. 21 Correlation analysis between mAbs productivity and SINEUP expression was assessed by dispersion analysis and Pearson's correlation index.
Differential In-Gel analysis (2D-DIGE)
Purified B9x mAb preparations from HEK293E or HEK293ES_1 expressing the SINEUP-40+32 cells were precipitated in 9:1 v/v acetone/methanol, at −20°C over night. Proteins were recovered by centrifugation at 16,000 x g at 4°C for 1 h. Total proteins were dissolved in 7 M urea, 2 M thiourea, 4% CHAPS. 22 µg of each mAb were labelled with Cy3 or Cy5 fluorescent dyes for 30 min. An equal amount of proteins from each sample was labelled with Cy2 as internal standard. 22 5 µg of each labelled sample were combined, supplemented with 7 M urea, 2 M thiourea, 4% CHAPS solution containing 130 mM DTT, 2% immobilized pH gradient (IPG) buffer (pH 3–10 NL) and 2.8% DeStreak reagent (GE Healthcare, Little Chalfont, UK) and used for passive hydration of immobilized pH gradient (IPG) strips (7 cm) to run the isoelectric focusing on a IPGphor II apparatus (GE Healthcare). After the first dimension, the strip was equilibrated and transferred onto 12% polyacrylamide gels for the second dimension. Gels were scanned by using Typhoon 9400 variable mode imager (GE Healthcare) using the following excitation/emission wavelengths: 488/520 for Cy2, 532/580 for Cy3 and 633/670 for Cy5. Images were acquired with the Image Quant software (GE Healthcare). The data were analysed by Differential in-Gel Analysis (DIA) of DeCyder software (GE Healthcare). 22
FACS analysis
Huh7.5 cells were detached by 5 mM EDTA in PBS. Cells were counted and plated at 5×105 cells/well in a 96 round bottom multiwell. Cells were stained for 20′ with LIVE/DEAD™ Fixable Violet Dead Cell Stain Kit (Invitrogen) according to manufacturer's instruction. After 1 wash in PBS, cells were incubated for 30 minutes at room temperature with 100 nM purified B9x mAb produced in HEK293E or HEK293ES_1 expressing the SINEUP-40+32 cells. After one wash with PBS, the cells were stained for 30 minutes at room temperature with Allophycocyanin (APC) AffiniPure F(ab')₂ Fragment Goat Anti-Human IgG, Fcγ Fragment Specific (Jackson ImmunoResearch, Catalog number: 109-136-098). After two washes with PBS, the cells were resuspended in 2.5 mM EDTA, and analyzed on CytoFLEX Flow Cytometer (Beckman Coulter) with Data Anal CytExpert software (Beckman Coulter).
Supplementary Material
Funding Statement
This work was supported by the European Commission, Grant FP7-HEALTH 305600, HepaMAb.
Abbreviations
- Ig
immunoglobulin
- lncRNA
long non-coding RNA
- mAb
monoclonal antibody
- PTM
post-translational modification
- scFv
single-chain variable fragment.
Disclosure of potential conflicts of interest
A.N. owns shares in ReiThera S.R.L.
Acknowledgments
The authors wish to thank Dr. Elisa Scarselli for helpful discussion and for critical reading.
References
- 1.Ecker DM, Jones SD, Levine HL. The therapeutic monoclonal antibody market. mAbs. 2015;7(1):9–14. doi: 10.4161/19420862.2015.989042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Köhler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–7. doi: 10.1038/256495a0. PMID:1172191. [DOI] [PubMed] [Google Scholar]
- 3.Frenzel A, Hust M, Schirrmann T. Expression of recombinant antibodies. Front Immunol. 2013;4:217. doi: 10.3389/fimmu.2013.00217. PMID:23908655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sheehan J, Marasco WA. Phage and yeast display. Microbiol Spectr. 2015;3(1):AID-0028-2014. doi: 10.1128/microbiolspec.AID-0028-2014. PMID:26104550. [DOI] [PubMed] [Google Scholar]
- 5.De Marco A. Recombinant antibody production evolves into multiple options aimed at yielding reagents suitable for application-specific needs. Microb Cell Fact. 2015;14:125. DOI 10.1186/s12934-015-0320-7. PMID:26330219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kunert R, Reinhart D. Advances in recombinant antibody manufacturing. Appl Microbiol Biotechnol. 2016;100:3451–61. DOI 10.1007/s00253-016-7388-9. PMID:26936774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dumont J, Euwart D, Mei B, Estes S, Kshirsagar R. Human cell lines for biopharmaceutical manufacturing: history, status, and future perspectives. Crit Rev Biotechnol. 2016;36(6):1110–22. doi: 10.3109/07388551.2015.1084266. PMID:26383226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Croset A, Delafosse L, Gaudry JP, Arod C, Glez L, Losberger C, Begue D, Krstanovic A, Robert F, Vilbois F et al. . Differences in the glycosylation of recombinant proteins expressed in HEK and CHO cells. J Biotechnol. 2012;161:336–348. DOI: 10.1016/j.jbiotec.2012.06.038. [DOI] [PubMed] [Google Scholar]
- 9.Higel F, Seidl A, Sörgel F, Friess W. N-glycosylation heterogeneity and the influence on structure, function and pharmacokinetics of monoclonal antibodies and Fc fusion proteins. Eur J Pharm Biopharm. 2016;100:94–100. doi: 10.1016/j.ejpb.2016.01.005. PMID:26775146. [DOI] [PubMed] [Google Scholar]
- 10.Young JM, Cheadle C, Foulke JS Jr, Drohan WN, Sarver N. Utilization of an Epstein-Barr virus replicon as a eukaryotic expression vector. Gene. 1988;62:171–85 [DOI] [PubMed] [Google Scholar]
- 11.Backliwal G, Hildinger M, Chenuet S, Wulhfard S, De Jesus M, Wurm FM. Rational vector design and multi-pathway modulation of HEK 293E cells yield recombinant antibody titers exceeding 1 g/l by transient transfection under serum-free conditions. Nucleic Acids Res. 2008;36(15):e96. doi: 10.1093/nar/gkn423. PMID:18617574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vink T, Oudshoorn-Dickmann M, Roza M, Reitsma JJ, de Jong RN. A simple, robust and highly efficient transient expression system for producing antibodies. Methods. 2014;65(1):5–10. doi: 10.1016/j.ymeth.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 13.Nishimiya D. Proteins improving recombinant antibody production in mammalian cells. Appl Microbiol Biotechnol. 2014;98(3):1031–42. DOI 10.1007/s00253-013-5427-3. [DOI] [PubMed] [Google Scholar]
- 14.Carrieri C, Cimatti L, Biagioli M, Beugnet A, Zucchelli S, Fedele S, Pesce E, Ferrer I, Collavin L, Santoro C, et al. . Long non-coding antisense RNA controls Uchl1 translation through an embedded SINEB2 repeat. Nature. 2012;491:454–7. doi: 10.1038/nature11508. PMID:23064229. [DOI] [PubMed] [Google Scholar]
- 15.Zucchelli S, Fasolo F, Russo R, Cimatti L, Patrucco L, Takahashi H, Jones MH, Santoro C, Sblattero D, Cotella D, et al. . SINEUPs are modular antisense long non-coding RNAs that increase synthesis of target proteins in cells. Front Cell Neurosci. 2015;9:174. doi: 10.3389/fncel.2015.00174. PMID:26029048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patrucco L, Chiesa A, Soluri MF, Fasolo F, Takahashi H, Carninci P, Zucchelli S, Santoro C, Gustincich S, Sblattero D, et al. . Engineering mammalian cell factories with SINEUP noncoding RNAs to improve translation of secreted proteins. Gene. 2015;569(2):287–93. doi: 10.1016/j.gene.2015.05.070. [DOI] [PubMed] [Google Scholar]
- 17.Yao Y, Jin S, Long H, Yu Y, Zhang Z, Cheng G, Xu C, Ding Y, Guan Q, Li N, et al. . RNAe: an effective method for targeted protein translation enhancement by artificial non-coding RNA with SINEB2 repeat. Nucleic Acids Res. 2015;43(9):e58. doi: 10.1093/nar/gkv125. PMID:2572236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paciello R, Urbanowicz RA, Riccio G, Sasso E, McClure CP, Zambrano N, Ball JK, Cortese R, Nicosia A, De Lorenzo C. Novel human anti-Claudin 1 monoclonal antibodies inhibit HCV infection and may synergize with anti-SRB1 mAb. J Gen Virol. 2016;97(1):82–94. doi: 10.1099/jgv.0.000330. PMID:26519290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nebija D, Noe CR, Urban E, Lachmann B. Quality Control and stability studies with the monoclonal antibody, Trastuzumab: Application of 1D- vs. 2D-Gel Electrophoresis. Int J Mol Sci. 2014;15:6399–411. doi: 10.3390/ijms15046399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sasso E, Paciello R, D'Auria F, Riccio G, Froechlich G, Cortese R, Nicosia A, De Lorenzo C, Zambrano N. One-step recovery of scFv Clones from high-throughput sequencing-based screening of phage display libraries challenged to cells expressing native Claudin-1. BioMed Res Int. 2015;2015:703213. doi: 10.1155/2015/703213. PMID:26649313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25:402–8 [DOI] [PubMed] [Google Scholar]
- 22.Monteleone F, Rosa R, Vitale M, D'Ambrosio C, Succoio M, Formisano L, Nappi L, Romano MF, Scaloni A, Tortora G, Bianco R, Zambrano N. Increased anaerobic metabolism is a distinctive signature in a colorectal cancer cellular model of resistance to antiepidermal growth factor receptor antibody. Proteomics. 2013;13(5):866–77. doi: 10.1002/pmic.201200303. PMID:23281225. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


