Figure 1. Strategies for α–functionalization of aliphatic amines.
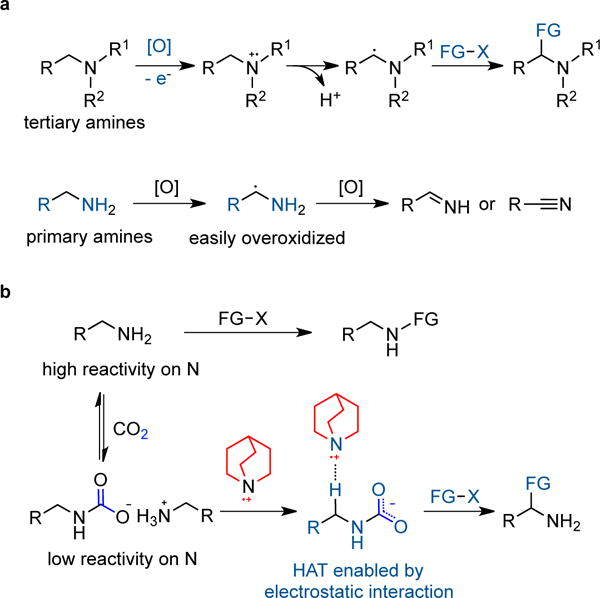
a, Generation and reactivity of α–amino radical from tertiary amines have been well establised but synthetic studies on α–amino radical of primary amines are rare as they can be easily overoxidized to imines or nitriles. b, Our hypothesis using CO2 as an activator for the α–functionalization of simple primary aliphatic amines relies on an electrostatic attraction between the in situ formed carbamate and quinuclidinium radical cation; CO2 also serves as a temporary protecting group to decrease the nucleophilicity of the NH2 group; counterion omitted for clarity. R, R1, R2, and X denote a general organic group. FG, functional group. HAT, hydrogen atom transfer.
